
Perovskites and related phases
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص616-617
الجزء والصفحة:
ص616-617
 2025-10-09
2025-10-09
 302
302
Perovskites and related phases
Key points: The perovskites have the general formula ABX3, in which the 12-coordinate hole of a ReO3 type BX3 structure is occupied by a large A ion; the perovskite barium titanate, BaTiO3, exhibits ferro electric and piezoelectric properties associated with cooperative displacements of the ions. The perovskites have the general formula ABX3, in which the 12-coordinate hole of BX3 (as in ReO3) is occupied by a large A ion (Fig. 24.19; a different view of this structure is given in Fig. 3.42) The X ion is most frequently O2 or F (as in NaFeF3) although nitride- and hydride-containing perovskites can also be synthesized, such as in LiSrH3. Perovskite itself is named after the naturally occurring oxide mineral CaTiO3 and the largest class of perovskites are those with the anion as oxide. This breadth of perovskites is widened by the observation that solid solutions and nonstoichiometry are also common features of the perovskite structure, as in Ba1-x Srx TiO3 and SrFeO3-y. Some metal-rich materials adopt the perovskite structure with the normal distribution of cations and anions partially inverted, for instance SnNCo3. The perovskite structure is often observed to be distorted such that the unit cell is no longer centrosymmetric and the crystal acquires an overall permanent electric polarization as a result of ion displacements. Some polar crystals are ferroelectric in the sense that they resemble ferromagnets, but instead of the electron spins being aligned over a region of the crystal, the electric dipole moments of many unit cells are aligned. As a result, the relative permittivity, which reflects the polarity of a compound, for a ferroelectric material often exceeds 1x103 and can be as high as 1.5x104; for comparison, the relative permittivity of liquid water is about 80 at room temperature. Barium titanate, BaTiO3, is the most extensively studied example of such a material. At temperatures above 120°C this compound has the perfect cubic perovskite structure. At room temperature, it adopts a lower symmetry, tetragonal unit cell in which the various ions can be considered as having been dis placed from their normal high symmetry sites (Fig. 24.20). This displacement results in a spontaneous polarization of the unit cell and formation of an electric dipole; coupling between these ion displacements and therefore the induced dipoles is very weak. Application

Figure 24.19 Views of the perovskite (ABO3) structure (a) emphasizing the 12-fold coordination of the larger A cation and showing the relationship with the ReO3 structure of Fig. 23.22b, (b) highlighting the octahedral coordination of the B cation. (c) A polyhedral representation accentuating the BO6 octahedra of an external electric field aligns these dipoles throughout the material, resulting in a bulk polarization in a particular direction, which can persist after removal of the electric field. The temperature below which this spontaneous polarization can occur and the material behaves as a ferroelectric is called the Curie temperature (TC)(Section 20.8). For BaTiO3 TC=120°C. The high relative permittivity of barium titanate leads to its use in capacitors, where its presence allows up to 1000 times the charge to be stored in comparison with a capacitor with air between the plates. The introduction of dopants into the barium titanate structure, forming solid solutions, allows various properties of the compound to be tuned. For example, the replacement of Ba by Sr or of Ti by Zr causes a sharp lowering of TC. Another characteristic of many crystals, including a number of perovskites that lack a centre of symmetry, is piezoelectricity, the generation of an electrical field when the crystal is under stress or the change in dimensions of the crystal when an electrical field is applied. Piezoelectric materials are used for a variety of applications, such as pressure transducers, ultra micromanipulators (where very small movements can be controlled), sound detectors, and as the probe support in scanning tunnelling microscopy. Some important examples are BaTiO3, NaNbO3, NaTaO3, and KTaO3.
Although a noncentrosymmetric structure is required for both ferroelectric and piezo electric behaviour, the two phenomena do not necessarily occur for the same crystal. For example, quartz, which does not have the perovskite structure, is permanently polarized; although quartz is piezoelectric it is not ferroelectric because an external electric field cannot reverse the polarization. Quartz is widely used to set the clock rate of microprocessors and watches because a thin sliver oscillates at a specific frequency to produce a small oscillating electrical field. This frequency is very insensitive to temperature. Another prototypical structure, that of potassium tetrafluoridonickelate(II), K2 NiF4 (Fig. 24.21), is related to perovskite. The compound can be thought of as containing individual slices from the perovskite structure that share the four F atoms from the octahedra within the layer and have terminal F atoms above and below the layer. These layers are displaced relative to each other and separated by the K ions (which are nine-coordinate, to eight F atoms of one layer and one terminal F atom from the next).
Compounds with the K2NiF4 structure have come under renewed investigation because some high-temperature superconductors, such as La1.85 Sr0.15 CuO4, crystallize with this structure. Apart from their importance in superconductivity, compounds with the K2NiF4 structure also provide an opportunity to investigate two-dimensional magnetic domains as coupling between electron spins is much stronger within the layers of linked octahedra than between the layers. The K2NiF4 structure has been introduced as being derived from a single slice of the perovskite structure; other related structures are possible where two or more perovskite layers are displaced horizontally relative to each other. Structures with K2NiF4 at one end of the range (a single perovskite layer) and perovskite itself at the other (an infinite number of such layers) are known as Ruddlesden–Popper phases. They include Sr3Fe2 O7 with double layers and Ca4Mn3O10 with triple layers (Fig. 24.22).
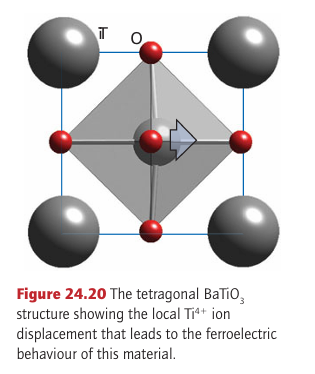
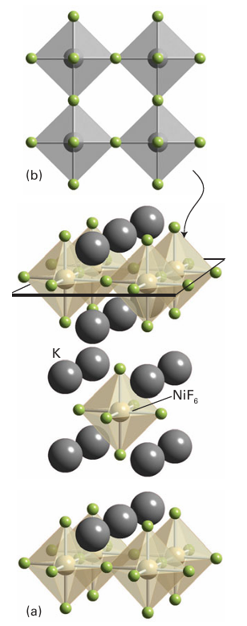
Figure 24.21 The K2NiF4 structure. (a) The displaced layers of NiF6 octahedra interspersed with K ions and (b) a view of one layer of composition NiF4 showing the corner sharing octahedra linked through F.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


