
Electronic properties
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
613
الجزء والصفحة:
613
 2025-10-09
2025-10-09
 258
258
Electronic properties
Key points: The 3d-metal monoxides MnO, FeO, CoO, and NiO are semiconductors; TiO and VO are metallic conductors. The 3d-metal monoxides MnO, Fe1-x O, CoO, and NiO have low electrical conductivities that increase with temperature (corresponding to semiconducting behaviour) or have such large band gaps that they are insulators. The electron or hole migration in these oxide semiconductors is attributed to a hopping mechanism. In this model, the electron or hole hops from one localized metal atom site to the next. When it lands on a new site it causes the surrounding ions to adjust their locations and the electron or hole is trapped temporarily in the potential well produced by this distortion. The electron resides at its new site until it is thermally activated to migrate into another nearby site. Another aspect of this charge hopping mechanism is that the electron or hole tends to associate with local defects, so the activation energy for charge transport may also include the energy of freeing the hole from its position next to a defect. Hopping contrasts with the band model for semiconductivity discussed in Section 3.20, where the conduction and valence electrons occupy orbitals that spread through the whole crystal. The difference stems from the less diffuse d orbitals in the monoxides of the mid-to-late 3d metals, which are too compact to form the broad bands necessary for metallic conduction. When NiO is doped with Li2O in an O2 atmosphere a solid solution Lix (Ni2+)1-2x (Ni3+) x O is obtained, which has greatly increased conductivity for reasons similar to the increase in conductivity of Si when doped with B. The characteristic pronounced increase in electronic conductivity with increasing temperature of metal oxide semiconductors is used in ‘thermistors’ to measure temperature. In contrast to the semiconductivity of the monoxides in the centre and right of the 3d series, TiO and VO have high electronic conductivities that decrease with increasing temperature. This metallic conductivity persists over a broad composition range from highly oxygen-rich Ti1 x O to metal-rich TiO1-x. In these compounds, a conduction band is formed by the overlap of the t2g orbitals of metal ions in neighbouring octahedral sites that are oriented towards each other (Fig. 24.11). The radial extension of the d orbitals of these early d-block elements is greater than for elements later in the period, and a band results from their overlap (Fig. 24.12); this band is only partly filled. The widely varying compositions of these monoxides also appear to be associated with the electronic delocalization: the conduction band serves as a rapidly accessible source and sink of electrons that can readily compensate for the formation of vacancies.
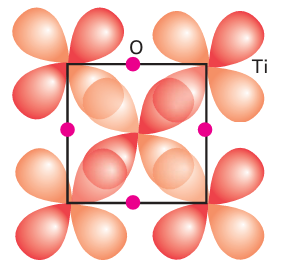
Figure 24.11 Overlap of the dzx orbitals in TiO to give a t2g band. In the perpendicular directions the dyx and dzy orbitals overlap in an identical manner.
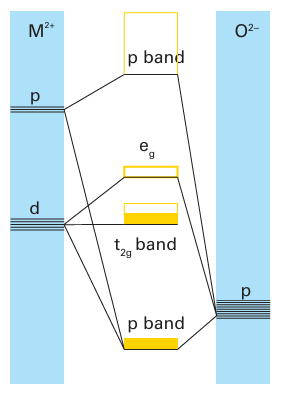
Figure 24.12 Molecular orbital energy level diagram for early d-metal monoxides. The t2g band is only partly filled and metallic conduction results.
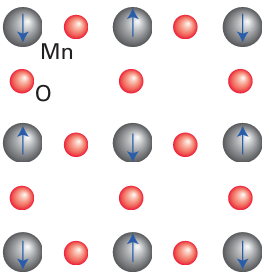
Figure 24.13 The arrangement of electron spins on the Mn2+ ions in the antiferromagnetic state of MnO.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


