
Defects and nonstoichiometry
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
612
الجزء والصفحة:
612
 2025-10-09
2025-10-09
 289
289
Defects and nonstoichiometry
Key point: The nonstoichiometry of Fe1–x Oarises from the creation of vacancies on the Fe2+ octahedral sites, with each vacancy charge-compensated by the conversion of two Fe2+ ions to two Fe3+ ions. The origin of nonstoichiometry in FeO has been studied in more detail than that in most other MO compounds. In fact, it is found that stoichiometric FeO does not exist but rather a range of iron-deficient compounds in the range Fe1 x O, 0.13 x 0.04, can be obtained by quenching (cooling very rapidly) iron (II) oxide from high temperatures. The compound Fe1-x O is in fact metastable at room temperature: it is thermodynamically unstable with respect to disproportionation into iron metal and Fe3O4 but does not convert for kinetic reasons. The general consensus is that the structure of Fe1-x O is derived from the rock-salt FeO structure by the presence of vacancies on the Fe2+ octahedral sites, and that each-vacancy is charge-compensated by the conversion of two adjacent Fe2+ ions to two Fe3+ ions. The relative ease of oxidizing Fe (II) to Fe (III) accounts for the fairly broad range of com positions of Fe1-x O. At high temperatures, the interstitial Fe3 ions associate with the Fe2+ vacancies (or defects) to form clusters distributed throughout the structure (Fig. 24.10). Similar defects and the clustering of defects appear to occur with all other 3d-metal monoxides, with the possible exception of NiO. The range of nonstoichiometry in Ni1-x O is extremely narrow, but conductivity and the rate of atom diffusion vary with oxygen partial pressure in a manner that suggests the presence of isolated point defects. Both CoO and NiO occur in a metal-deficient state, although their range of compositions is again not as broad as that of FeO. As indicated by standard potentials in aqueous solution, Fe (II) is more easily oxidized than either Co (II) or Ni (II); this solution redox chemistry correlates well with the much smaller range of oxygen deficiency in NiO and CoO. Chromium (II) oxide, like FeO, spontaneously disproportionates:
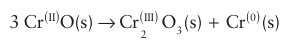
However, the material can be stabilized by crystallization in a copper(II) oxide matrix. Both CrO and TiO have structures that show high levels of defects on both the cation and anion sites forming metal-rich or metal-deficient stoichiometries (Ti1-x O and TiO1-x). In fact, TiO has large numbers of vacancies, in equal amounts, on both cation and anion sublattices, rather than the expected perfect, defect-free structure. Note that significant deviations from the stoichiometry MO are out of the question for Group 2 metal oxides, such as CaO, because for these elements M3+ ions are chemically inaccessible.
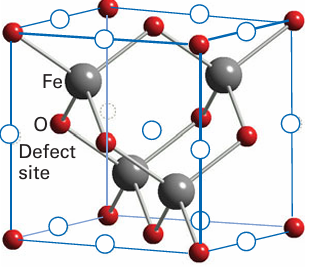
Figure 24.10 Defect sites proposed for Fe1-x O. Note that the tetrahedral Fe3+ interstitials (grey spheres) and octahedral Fe2+ vacancies (circles) are clustered together.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


