
Mixed ionic-electronic conductors
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص610-611
الجزء والصفحة:
ص610-611
 2025-10-09
2025-10-09
 221
221
Mixed ionic-electronic conductors
Key point: Solid materials can exhibit both ionic and electronic conductivity. Most ionic conductors, such as sodium ßn-alumina and YSZ, have low electronic conductivity (that is, conduction by electron rather than ion motion). Their application as solid electrolytes, in sensors for instance, requires this feature to avoid shorting-out the cell. In some cases a combination of electronic and ionic conductivity is desirable, and this type of behaviour can be found in some d-metal compounds where defects allow O2 conduction and the metal d orbitals provide an electronic conduction band. Many such materials are perovskite-based structures with mixed oxidation states at the B cation sites (Section 3.9). Two examples are La1-x Srx CoO3-y and La1-x Srx FeO3-y. These oxide systems are good electronic conductors with partially filled bands as a result of the nonintegral d-metal oxidation number and can 1The symbol S denotes siemens; 1 S=1Ω-1, where 1 Ω (ohm)=1 V A-1.
2The signal from this sensor is used to adjust the air/fuel ratio and thereby the composition of the exhaust gas being fed to the catalytic converter.
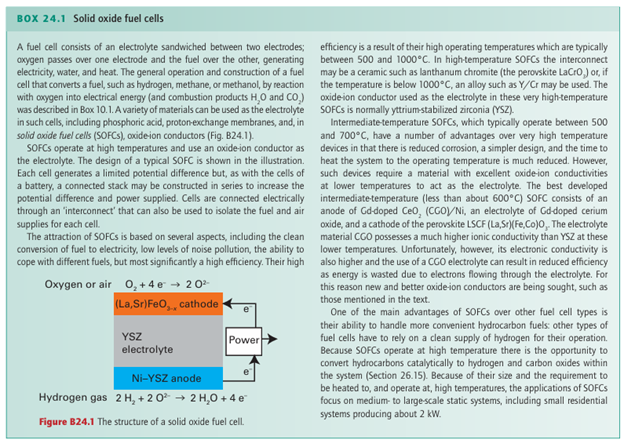
conduct by O2- migration through the perovskite O2- ion sites. This type of material is of use in solid oxide fuel cells (SOFC, Box 24.1), one type of fuel cell mentioned in Box 5.1, in which one electrode has to allow diffusion of ions through a conducting electrode.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


