
Extended defects
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص 605-606
الجزء والصفحة:
ص 605-606
 2025-10-08
2025-10-08
 299
299
Extended defects
Key point: Wadsley defects are shear planes that collect defects along certain crystallographic directions. Such defects entail a significant local distortion of the structure and in some instances localized charge imbalances too. Therefore, it should not be surprising that defects may cluster together and sometimes form lines and planes. Tungsten oxides illustrate the formation of planes of defects. As illustrated in Fig. 24.1, the idealized structure of WO3 (which is usually referred to as the ‘ReO3 structure’; see below) consists of WO6 octahedra sharing all vertices. To picture the formation of the defect plane, we imagine the removal of shared O atoms along a diagonal. Then adjacent slabs slip past each other in a motion that results in the completion of the vacant coordination sites around each W atom. This shearing motion creates edge-shared octahedra along a di agonal. The resulting structure was named a crystallographic shear plane by A.D. Wadsley, who first devised this way of describing extended planar defects. Crystallographic shear planes randomly distributed in the solid are called Wadsley defects. Such defects lead to a continuous range of compositions, as in tungsten oxide, which ranges from WO3 to WO2.93 (made by heating and reducing WO3 with tungsten metal). If, however, the crystallographic shear planes are distributed in a nonrandom, periodic manner, so giving rise to a new unit cell, then we should regard the material as a new stoichio metric phase. Thus, when even more O2- ions are removed from tungsten oxide, a series
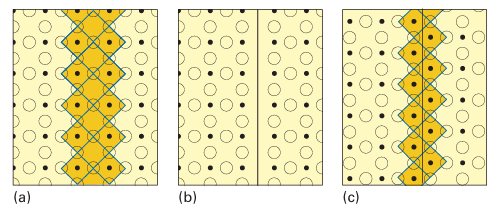
Figure 24.1 The concept of a crystallographic shear plane illustrated by the (100) plane of the ReO3 structure. (a) A plane of metal, Re, and oxygen, O, atoms. The octahedron around each metal atom is completed by a plane of oxygen atoms above and below the plane illustrated here. Some of the octahedra are shaded to clarify the processes that follow. (b) Oxygen atoms in the plane perpendicular to the page are removed, leaving two planes of metal atoms that lack their sixth oxygen ligand. (c) The octahedral coordination of the two planes of metal atoms is restored by translating the right slab as shown. This creates a plane (labelled a shear plane) vertical to the paper in which the MO6 octahedra share edges.
of discrete phases having ordered crystallographic shear planes and compositions WnO3n-2 (n=20, 24, 25, and 40) are observed. Compounds with closely spaced compositions that contain shear planes are known for oxides of W, Mo, Ti, and V and some of their complex oxides, for example the tungsten bronzes M8W9O47, M Nb, Ta and the ‘Magnéli phases’, VnO2n-1 (n=3-9). Electron microscopy (Section 25.3) provides an excellent method of observing these defects experimentally because it reveals both ordered and random arrays of shear planes (Fig. 24.2).
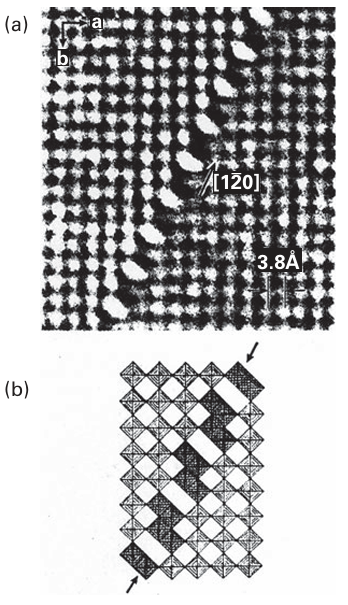
Figure 24.2 (a) High resolution electron micrograph lattice image of a crystallographic shear plane in WO3-x (b) The oxygen octahedral polyhedra that surround the W atoms imaged in the electron micrograph. Note the edge-shared octahedra along the crystallographic shear plane. [Reproduced by permission from S. Iijima, J. Solid State Chem. 1975, 14, 52].
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


