
Thorium and uranium
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص595-596
الجزء والصفحة:
ص595-596
 2025-10-07
2025-10-07
 306
306
Thorium and uranium
Key points: The common nuclides of thorium and uranium exhibit only low levels of radioactivity, so their chemical properties have been extensively developed; the uranyl cation is found in complexes with many different ligand donor atoms; the organometallic compounds of the elements are dominated by pentamethylcyclo-pentadienyl complexes.
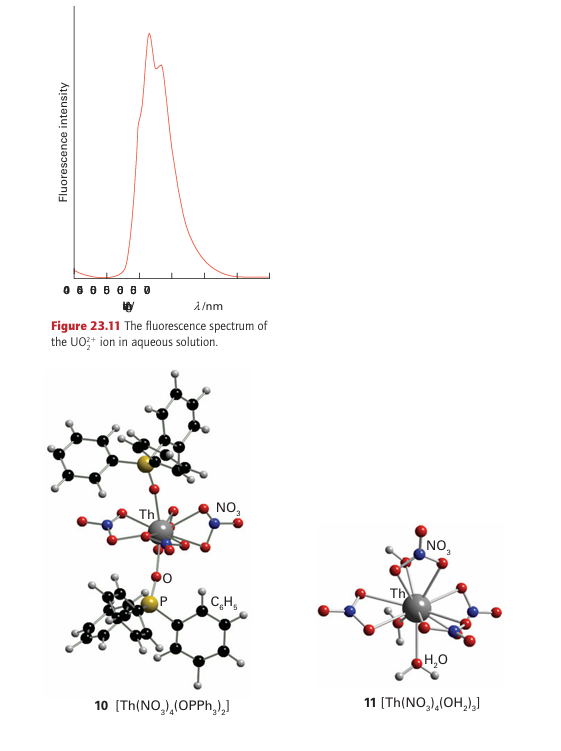
Because of their ready availability and low level of radioactivity, the chemical manipulation of Th and U can be carried out with ordinary laboratory techniques. As indicated in Fig. 23.9, the most stable oxidation state of Th in aqueous solution is Th (IV). This oxidation state also dominates the solid-state chemistry of the element. Eight-coordination is common in simple Th (IV) compounds. For example, ThO2 has the fluorite structure (in which a Th atom is surrounded by a cubic array of O-2 ions) and in ThCl4 and ThF4 the coordination numbers are also 8 with dodecahedral and square antiprism symmetry, respectively. The coordination number of Th in [Th (NO3)4 (OPPh3)2] is 10 (10), with the NO-3 ions and triphenylphosphine oxide groups arranged in a capped cubic array around the Th atom. The very unusual coordination number of 11 is exhibited by Th in its hydrated nitrate Th (NO3)4 .5H2O with the Th4 ion coordinated to four NO3 ions in a bidentate fashion and with three H2O molecules (11). The chemical properties of U are more varied than those of Th because the element has access to oxidation states from U(III) to U(VI), with U(IV) and U(VI) the most common. Uranium halides are known for the full range of oxidation states U(III) to U(VI), with a trend towards decreasing coordination number with increasing oxidation number. The U atom is nine-coordinate in solid UCl3, eight-coordinate in UCl4, and six-coordinate for the U(V) and U(VI) chlorides U2Cl10 (Fig 23.12) and UCl6, both of which are molecular compounds. The high volatility of UF6 (it sublimes at 57˚C) together with the occurrence of fluorine in a single isotopic form account for the use of this compound in the separation of the uranium isotopes by gaseous diffusion or centrifugation. In gas diffusion, the lighter 235UF6 molecules travel at greater average speeds and strike the walls of a container
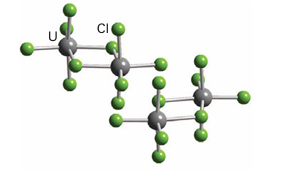
Figure 23.12 The crystal structure of U2Cl10 consists of discrete molecules formed from pairs of edge-sharing UCl6 octahedra.
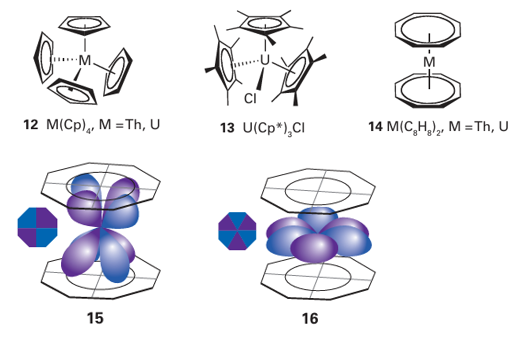
more frequently than their heavier isotopologues such as 238UF6. If the container-wall is permeable, 235UF6 will diffuse through it slightly more rapidly. A series of such ‘diffusers’, sometimes numbering thousands, enables enrichment of the gas in 235UF6. Uranium metal does not form a passivating oxide coating, so it becomes corroded on prolonged exposure to air to give a complex mixture of oxides. These oxides include UO2, U3O8, and several polymorphs of the stoichiometry UO3. The dioxide UO2 adopts the fluorite structure but also takes up interstitial O atoms to form the non-stoichiometric series UO2+x, 0<x<0.25. One form of uranium trioxide, Ϭ-UO3, adopts the ReO3 structure type (Section 24.8b). The most important oxide is UO3, which dissolves in acid to give the uranyl ion, UO2+2 [O=U=O]2+. In water, this ion forms complexes with many anions, such as NO3 and SO4+2. In contrast to the angular shape of the VO2+ ion and similar d0 complexes, the AnO2+n unit, with An=U, Np, Pu, and Am, maintains its linearity in all complexes. Both f-orbital bonding and relativistic effects have been invoked to explain this linearity. Compounds containing the UO2+2 ion show (2+n)-coordination, n=4, 5, and 6, with the four to six additional ligands forming a planar or near planar arrangement around the uranyl unit. Thus, the structure of UO2F2 has a slightly puckered ring of six F ions around the UO2+2 unit. The stability of the UO2+2 dication means that in most reactions it remains inert; recently, however, it has been shown that it is possible to reduce this species, when trapped within a rigid framework, to produce [O=U(V)-OR] +. The separation of U from most other metals is accomplished by the extraction of the neutral uranyl nitrato complex [UO2(NO3)2 (OH2)4] from the aqueous phase into a polar organic phase, such as a solution of tributyl phosphate dissolved in a hydrocarbon solvent. This kind of solvent-extraction process is used to separate actinoids from other fission products in spent nuclear fuel. The organometallic chemistry of U and Th is reasonably well developed and shows many similarities to that of the lanthanoids, except that Th and U occur in a number of oxidation states and are larger than the typical Ln ion. Thus, compounds are dominated by those containing good donor ligands, such as -bonded alkyl and cyclopentadienyl groups. The increased size of Th and U compared with typical lanthanoids means that the tetrahedral species Th (Cp)4 and U(Cp)4 (12) can be isolated as monomers, and not only can U(Cp*)3 be isolated but so too can U(Cp*)3 Cl (13). As with lanthanoid organometallic compounds, actinoid organometallic compounds do not obey the 18-electron rule (Section 22.1). Sandwich compounds are possible with the n8-cyclooctatetraene ligand, and both thorocene, Th (C8H8 )2 and uranocene, U (C8H8)2 (14) are known and are sufficiently stable not to react with water. Compared with lanthanoid complexes of cyclooctatetraene, the bonding in uranocene and thorocene is complicated by the extension of the f orbitals beyond the core of the atom. In theory, therefore, not only is bonding possible (15), but so is φ bonding (16). Quite how much of a role φ bonds play in the bonding in uranocene or thorocene is the subject of considerable debate, but it is interesting to note that actinoid cyclooctatetraene compounds are the only ‘real’ compounds (as opposed to metal dimers in the gas phase) that might contain any contribution from φ bonding.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


