
Electronic spectra
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص594-595
الجزء والصفحة:
ص594-595
 2025-10-07
2025-10-07
 293
293
Electronic spectra
Key points: The electronic spectra of the early actinoids have contributions from ligand-to-metal charge transfer, 5f → 6d, and 5f → 5f transitions. The uranyl ion fluoresces strongly. Transitions between electronic states involving only the f orbitals, the 5f and 6d orbit als, and ligand-to-metal charge transfer (LMCT) are all possible for the actinoid ions. The f-f transitions are broader and more intense than for the lanthanoids because the 5f orbitals interact more strongly with the ligands. Their molar absorption coefficients typically lie in the range 10-100 dm3 mol−1 cm−1. The most intense absorptions are associated with LMCT transitions. For instance, LMCT transitions result in the intense yellow colour of the uranyl ion, UO2+2, in solution and its compounds. In species such as U3 (f3) transitions such as 5f26d1← 5f3 occur at wavenumbers between 20 000 and 33 000 cm−1 (500 300 nm) giving solutions and compounds of this ion a deep orange red colour. For Np3+ and Pu3+, with increasing effective nuclear charge, the separation of the 5f and 6d levels increases and the corresponding transitions move into the UV region of the spectrum; Np3 solutions are violet and those of Pu3 are light violet blue due mainly to f-f transitions.
The uranyl ion, UO2+2, is also strongly fluorescent, with a strong emission between 500 and 550 nm on excitation with UV radiation (Fig. 23.11). This property has in the past been used for colouring glass, where the addition of 0.5-2 per cent of uranyl salts pro-duces a bright golden yellow colour. The glass has a bright, green yellow fluorescence in sunlight which adds to its attractiveness; when viewed under UV radiation it glows an intense green. However as this glass is radioactive its commercial production has been largely phased out in recent years.
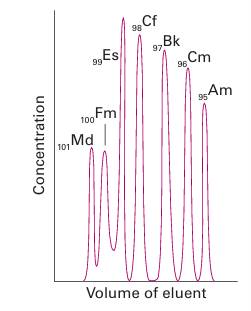
Figure 23.10 Elution of heavy actinoid ions from a cation exchange column using ammonium 2-hydroxyisobutyrate as the eluent. Note the similarity in elution sequence to Fig. 23.8: the heavy (smaller) An+3ions elute first.
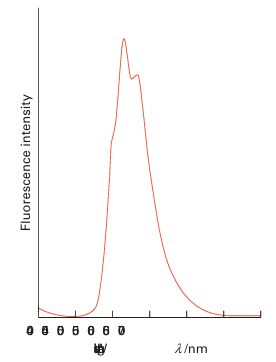
Figure 23.11 The fluorescence spectrum of the UO2+2 ion in aqueous solution.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


