
Organometallic compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص590-592
الجزء والصفحة:
ص590-592
 2025-10-07
2025-10-07
 270
270
Organometallic compounds
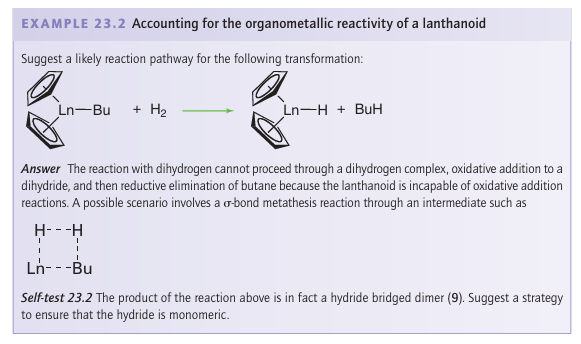
Key points: The organometallic compounds of the lanthanoids are dominated by good donor ligands, with complexes of acceptor ligands being rare; the bonding in complexes is best treated on the basis of ionic interactions; there are similarities between the organometallic compounds of the lanthanoids and those of the early d metals. In line with the picture of the lanthanoid ions as having no directional frontier orbitals, it should not be surprising that they do not exhibit a rich organometallic chemistry. In particular, the lack of any orbitals that can back bond to organic fragments (because the 5d orbitals are empty and the 4f orbitals are buried) restricts the number of bonding modes that are available. In addition, the strongly electropositive nature of the lanthanoids means that they need good donor, not good acceptor, ligands. Thus alkoxide, amide, and halide ligands, which are both and π donors, are common, whereas CO and phosphine ligands, which are both donors and π acceptors, and which do such a good job in stabilizing low oxidation state d-metal complexes, are rarely seen in lanthanoid chemistry. Indeed, under normal laboratory conditions, neutral metal carbonyl compounds are unknown for the f-block elements. As an example of the contrasting behaviour of organometallic complexes of the d and f blocks it should be noted that the first 2-alkene complex of a lanthanoid was characterized only in 1987, a century and a half after the first d-metal alkene complex, Zeise’s salt, was isolated. This lanthanoid alkene complex, [(Cp*)2 Yb(C2H4) Pt (PPh3)2] (4), has a particularly electron-rich alkene as it is already bound to an electron-rich Pt (0) centre, and it is thought that the alkene therefore needs no backbonding from the Yb atom to stabilize it. The bonding in the organometallic lanthanoid complexes that does form is predominantly ionic and is governed by electrostatic factors and steric requirements. Consequently there is no need for the 18-electron rule to be obeyed. Although there is a chemical uniformity across the f block, there is also a gradation of change with steric factors tending to dominate: a small change in ligand size can make a significant difference to reactivity. Owing to the weakness of the bonds, the lanthanoid complexes remain strong Lewis acids and the complexes are therefore very sensitive to air and moisture. The first organometallic compounds of the lanthanoids were cyclopentadienyl com pounds: G. Wilkinson made a large number of Ln(Cp)3 compounds with a wide variety of electron counts in 1954. The large lanthanoid ions can easily accommodate three cyclopentadienyl ligands, and they even tend to oligomerize, indicating that there is yet more space for additional ligands. Compounds of substituted cyclopentadienyl ligands are possible but the limit seems to have been reached with the sterically demanding pentameth-ylcyclopentadienyl (Cp*) ligand. It was, however, nearly 40 years after the original Ln (Cp)3 compounds were isolated that Ln (Cp*)3 compounds were obtained, and even then, they exist in equilibrium:
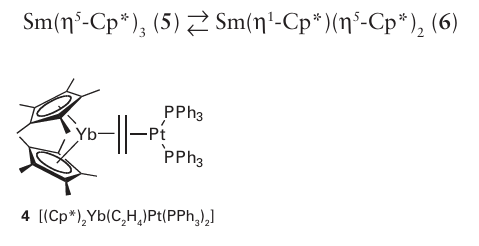
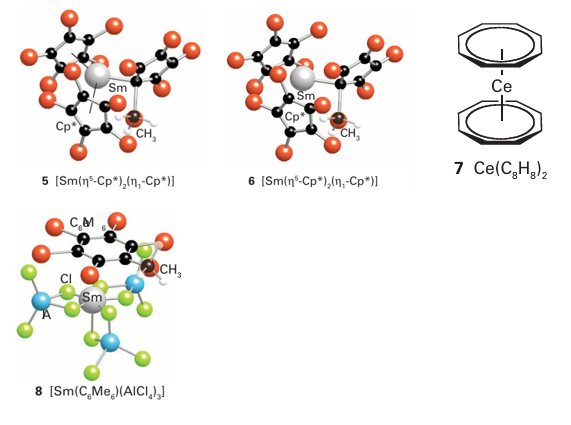
The great majority of lanthanoid organometallic compounds formally contain Ln (III) with a limited number of Ln (II) compounds: no other oxidation states are known. -Bonded alkyl groups are common, with compounds containing the cyclopentadienyl ligands tending to dominate. It is best to consider cyclopentadienyl compounds as containing Cp groups electrostatically bound to a central Ln3+ (or Ln2+) cation; this view is supported by the observation that the La compounds are diamagnetic. Com pounds containing 8-cyclooctatetraene ligands are known, such as Ce (C8H8)2 (7), and, as noted in Section 22.11, it is best to consider these to be complexes of the electron-rich C8H8-2 ion as ligand. Current research into lanthanoid organometallic compounds typically involves com pounds of the type [(Cp)2LnR]2, [(Cp)2LnR(sol)], [(Cp*) LnRX(sol)], and [(Cp*) LnR2 (sol)]. A number of arene complexes are also known, such as [(C6Me6) Sm (AlCl4)3] (8), where it is thought that the bonding is largely the result of an electrostatically induced dipole between the Sm3 ion and the electron-rich ring.
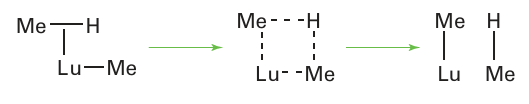
As well as an extensive organometallic chemistry of Ln (III) there are a number of Ln (II) molecular complexes that have been synthesized, including several for lanthanoids that rarely exist in this oxidation state. The species Er (II), Sm (II), and Yb (II) have extensive chemistries of this type, for example the compounds Ln (Cp*)2, but compounds of Tm (II), Dy (II), and Tm (II) have also been obtained, including TmI2 (DME)3. No lanthanoid has two stable states with oxidation numbers differing by 2, so there is no possibility of oxidative addition or reductive elimination reactions: -bond metathesis-type reactivity dominates (Section 22.23). In addition to the handling problems caused by the extreme sensitivity to air and moisture of the lanthanoid organometallic compounds, their study by NMR has been inhibited by the fact that they are all paramagnetic. Useful comparisons can be drawn through the study of compounds of the 4d-metal yttrium because Y3 has the same charge as and a size similar to a typical Ln3 ion, but is diamagnetic. In addition, yttrium is 100 per cent 89Y with I=1/2, so it is possible to measure Y-C and Y-H coupling constants quite easily and thus get additional structural information. There are strong similarities between the chemical properties of the early d-block organometallic compounds (those of Groups 3 to 5) and those of the f block. These similarities are to be expected because the early d metals are also strongly electropositive, have a limited number of d electrons to back bond with ligands, and have a limited number of accessible oxidation states. An example of a lanthanoid dinitrogen complex is (μ-N2) [(C5Me4H)2 La (THF)]2. Although the organometallic chemistry of the lanthanoids is less rich than that of the d metals, there are some striking examples of unusual reactions. For instance, a lanthanoid organometallic compound can be used for the activation of the C-H bond in methane. This discovery was based on the observation that 13CH4 exchanges 13C with the CH3 group attached to Lu:
This reaction can be carried out in deuterated cyclohexane with no evidence for activation of the cyclohexane C D bond, presumably because cyclohexane is too bulky to gain access to the Lu atom. A mechanism involving a four-centre -bond metathesis-type inter mediate has been proposed (Section 22.23):
Lanthanoid complexes are used in the Ziegler Natta polymerization of alkenes (Section 26.16). Dinitrogen complexes of lanthanoids were first reported in 1988.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


