
Binary ionic compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص586-587
الجزء والصفحة:
ص586-587
 2025-10-07
2025-10-07
 288
288
Binary ionic compounds
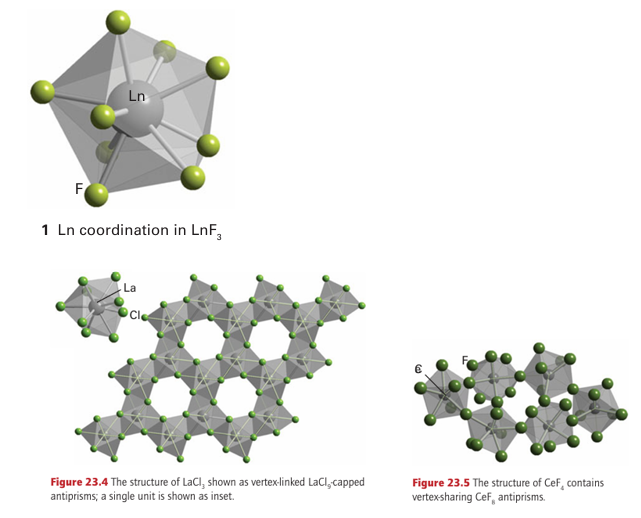
Key points: The structures of ionic lanthanoid compounds are determined by the size of the lanthanoid ion; binary oxides, halides, hydrides, and nitrides are all known. Lanthanoid (III) ions have radii that vary between 116 and 98 pm; for comparison, the ionic radius of Fe3 is 64 pm. Thus, the volume occupied by a Ln+3 ion is typically four to five times that occupied by a typical 3d-metal ion. Unlike the 3d metals, which rarely exceed a coordination number of 6 (with 4 being common too), compounds of lanthanoids often have high coordination numbers, typically between 6 and 12, and a wide variety of coordination environments. The binary lanthanoid (III) oxides, Ln2O3, have moderately complex structures with the coordination number of the Ln+3 ions being typically 7 (or a mixture of 6 and 7). Several related structure types termed A-, B-, C-Ln2 O3 are known and many of the oxides are poly morphic with transitions between the structures occurring as the temperature is changed. The coordination geometries are determined by the radius of the lanthanoid ion, with the average cation coordination number in the structures decreasing with decreasing ionic radius, for example the La3 ion in La2O3 has coordination number 7, whereas the Lu3 ion in Lu2O3 has coordination number 6. In cases where Ln4 ions can be obtained (for example with Ce, Pr, and Tb), the LnO2 adopts the fluorite structure as expected from radius-ratio rules (Section 3.10). Sulfides of stoichiometry LnS may be obtained by direct reaction of the elements at 1000°C and adopt the rock-salt structure. Phases of composition Ln2S3 can also be obtained by reaction of the lanthanoid trichloride with H2S; they have been studied as replacements for the toxic CdS and CdSe as possible pigments due to their intense red-orange-yellow colours.
The lanthanoid (III) trihalides have complex structural characteristics as a result of the high coordination numbers for these large ions. For example, in LaF3 the La3+ ion is in an irregular 11-coordinate environment and in LaCl3 it is in a nine-coordinate, capped anti-square prismatic environment (Fig. 23.4). Towards the end of the series the trihalides of the smaller lanthanoids have different structure types with lower coordination numbers for the same halide, as expected in view of the decrease in ionic radius. In LnF3, Ln has a nine-coordinate environment which can be considered as a capped anti-square prismatic environment distorted or a tricapped trigonal prism (1) and the compounds LnCl3 have layer structures based on six-coordinate Ln in a cubic close-packed array of Cl ions. Cerium is the only lanthanoid to form a tetrahalide (CeF4); it crystallizes with a structure formed from vertex-sharing CeF8 polyhedra (Fig. 23.5).
All the lanthanoids form hydrides of stoichiometry LnH2 which adopt the fluorite structure (Section 3.9a) based on cubic close-packed H ions with lanthanoid ions in all the tetrahedral holes. Cerium hydride may be further oxidized by hydrogen to form a series of nonstoichiometric phases of formula CeH2+x , with additional H ions incorporated into the fluorite lattice. Some of the smaller lanthanoids (for instance Dy, Yb, and Lu) form stoichio metric trihydrides, LnH3. Complex metal hydrides containing lanthanum, such as LaNi5 H6, have been studied intensively as possible hydrogen-storage materials, Box 10.4 and Sections 24.14, 24.15, as they can be prepared by heating the alloy LaNi5 in hydrogen gas under pressure; the reverse reaction occurs when LaNi5 H6 is heated under ambient pressure conditions evolving H2 gas.
Nitrides of composition LnN exist for all the lanthanoids and adopt the expected rock salt structure with alternating Ln3 and N3 ions. Three different lanthanoid carbide stoichiometries are known: M3C, M2C3, and MC2 . The M3 C phases form for the heavier lanthanoids and contain isolated carbon atoms and are hydrolysed by water to produce methane. The M2C3 phases form for the lighter lanthanoids La Ho and contain the di carbide anion C22-, as is also found in CaC2 (Section 3.9). The MC2 phases show metallic properties and except for the elements that form stable divalent cations, such as Yb, they can be expressed as Ln3 (C22−, e−) with the electron in a conduction band formed from Ln6s
and Ln6p orbital overlap; they react with water to form ethyne and other hydrocarbons. The lanthanoid nickel borocarbides, LnNi2B2C, have structures containing alternating layers of the stoichiometries LnC and Ni2B2. These borocarbides are superconductors at low temperature: the transition temperature for LuNi2B2C, for instance, is 16 K.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


