
General trends
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص 582-583
الجزء والصفحة:
ص 582-583
 2025-10-06
2025-10-06
 327
327
General trends
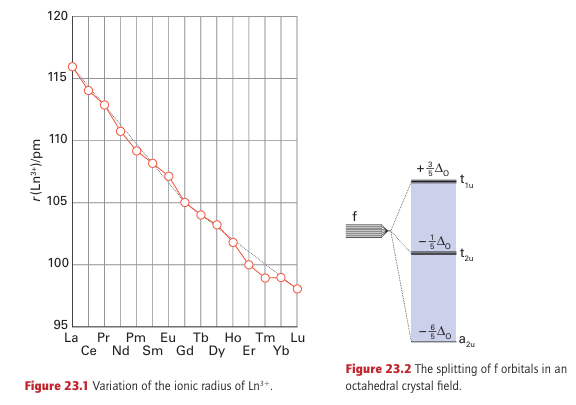
Key points: The lanthanoids are electropositive metals that commonly occur in their compounds as Ln (III); other oxidation states are stable only when an empty, half-filled, or full f subshell is produced. The elements La through to Lu are all highly electropositive, with standard potentials of the Ln3+ /Ln couple similar to that of Mg2+ /Mg (Table 23.2). They favour the oxidation state Ln (III) with a uniformity that is unprecedented in the periodic table. Other properties of the elements vary significantly. For example, the radii of the Ln3+ ions contract steadily from 116 pm for La3 to 98 pm for Lu3+. The decrease in ionic radius is attributed in part to the increase in the effective atomic number, Zeff, as electrons are added to the poorly shielding 4f subshell (Section 1.9), but detailed calculations indicate that subtle relativistic effects also make a substantial contribution. Figure 23.1 shows that the decrease in ionic radius corresponds to two gentle curves intersecting at Gd3 (f7). The effect is similar to that in the d block and can be traced to the splitting of the f-orbital energies by the crystal field generated by the ligand environment (Fig. 23.2), but the splitting is much smaller than in the d block. On the left of the series, the lower energy f orbitals (which point away from the ligands) are occupied as the number of f electrons increases (f1 to f4). For later configurations, such as f6 and f7, the higher energy
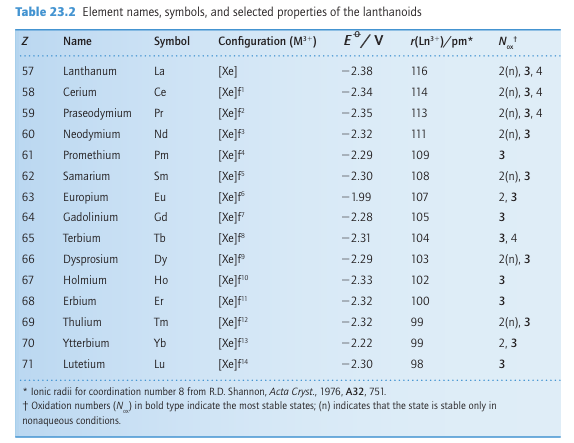
forbitals are occupied, and as these point towards the ligands the increased electron ligand repulsion results in a smaller than expected decrease in ionic radius. The 18 per cent decrease in ionic radius from La3 to Lu3 leads to an increase in the hydration enthalpy across the series. The standard potentials of the lanthanoids are all very similar, with EO (La3+ /La) 2.38 V similar to EO (Lu3+ /Lu) 2.30 V at the other end of the series. This similarity reflects the balance of increasing hydration and atomization enthalpies as the atomic radius decreases. The common occurrence of the lanthanoids as Ln (III) is normally ascribed to the fact that once the valence s and d electrons have been removed the f electrons are held tightly by the nucleus and do not extend beyond the xenon-like core of the atom. A further consequence of the burying of the f electrons is that a Ln3+ ion has no frontier orbitals with directional preference, so ligand-field stabilization plays only a small part in the proper ties of lanthanoid complexes. For a few complexes weak stabilization energies (of a few kilojoules per mole) are observed for configurations such as f4, f10, and f11 when compared with f0, f7, and f14. This stabilization can be explained following similar arguments to those described in Section 20.1 but using f orbitals and acknowledging the much weaker effects of the ligand field.
Superimposed on the common occurrence of Ln3 there are some atypical oxidation states that are most prevalent when the ion can attain the relatively more stable empty (f0), half filled (f7), or filled (f14) subshell (Table 23.2). Thus, Ce3+ (f1) can be oxidized to Ce4 (f0) and the latter is a strong and useful oxidizing agent. The next most common of the atypical oxidation states is Eu2 (f7), and there are a number of stable Eu2 compounds, including EuI2, EuSO4, and EuCO3, and solutions of this ion are stable. The ions Sm2 and Yb2 also have an extensive chemistry but reduce water to hydrogen in aqueous solutions. Recently Dy (II), Nd (II), and Tm (II) have been produced in molecular complexes in solution; an example is NdI2 (THF)5. Other reasonably stable oxidation states include Pr (IV) and Tb (IV), and the ox ides of these elements formed in air, Pr6O11 and Tb4O7, contain mixtures of Ln (III) and Ln (IV). Under very strongly oxidizing conditions Dy(IV) and Nd(IV) can be obtained. A Ln3 ion is a hard Lewis acid, as indicated by its preference for F and oxygen containing ligands and its occurrence with PO4+3 in minerals.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


