
1,1-Migratory insertion reactions
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
573-574
الجزء والصفحة:
573-574
 2025-10-06
2025-10-06
 363
363
1,1-Migratory insertion reactions
Key point: 1,1-Migratory insertion reactions result from the migration of a species such as a hydride or alkyl group to an adjacent ligand such as carbonyl to give a metal complex with two fewer electrons on the metal atom. A1,1-migratory insertion reaction is exemplified by reactions of the 1-CO ligand, where the following change can take place:

The reaction is called a ‘1,1-reaction’ because the X group that was one bond away from the metal atom ends up on an atom that is one bond away from the metal atom. Typically the X group is an alkyl or aryl species and then the product contains an acyl group. In principle, the reaction could proceed by a migration of the X group, or an insertion of the CO into the M-X bond. An uncertainty about the actual mechanism has led to the apparently contradictory name migratory insertion. Colloquially, however, the terms ‘migratory insertion’, ‘migration’, and ‘insertion’ are used interchangeably. The overall reaction results in a decrease in the number of electrons on the metal atom by2, with no change in the oxidation state. It is therefore possible to induce 1,1-migratory insertion reactions by the addition of another species that can act as a ligand:
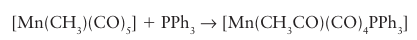
The classic study of the migratory insertion of CO with [CH3Mn (CO)5] illustrates a number of key features of reactions of this type.4 First, in the reaction
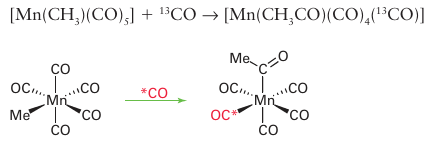
the product has only one labelled CO, and that group is cis to the newly formed acyl group. This stereochemistry demonstrates that the incoming CO group does not insert into the Mn-CH3 bond, and that either the methyl group migrates to an adjacent CO ligand, or a CO ligand adjacent to the methyl group inserts into the Mn CH3 bond. Second, in the reverse reaction
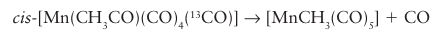
it is possible to distinguish between the migration of the methyl group and the insertion of the CO ligand: cis- [Mn (CH3CO) (CO)4 (13CO)] must lose a CO ligand cis to the acyl group in order for the reaction to proceed. The scheme below summarizes the potential reaction pathways. In one quarter of the instances, this ligand will be the labelled CO and there will be no significant information gained. In half the instances, a nonlabelled CO will be lost, leaving a vacant site cis to both the labelled CO ligand and the acyl group. In this case, either (a) migration of the methyl group back to the metal atom or (b) extrusion of CO will lead to the methyl group and the 13CO ligand being cis to each other and no information is gained. However, in the remaining one quarter of the instances, the CO that is trans to the labelled CO ligand will be lost, and in this case it is possible to distinguish (c) CO ligand extrusion from (d) methyl group migration. If the methyl group migrates, it ends up trans to the labelled CO, whereas if the CO is extruded, the methyl group ends up cis to the CO.
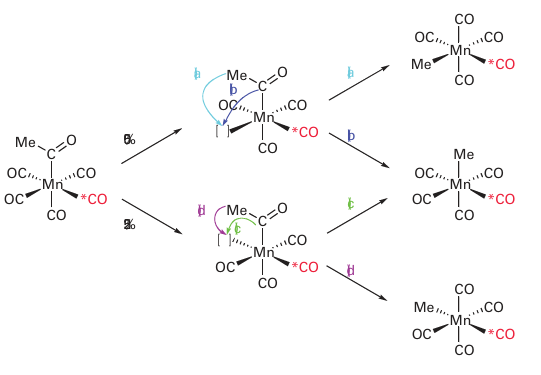
Because the product with CH3 and 13COtrans to each other constitutes about 25 per cent of the product, we can conclude that the CH3 group does indeed migrate. Application of the principle of microscopic reversibility5 allows us to conclude that the forward reaction proceeds by methyl-group migration. All 1,1-migratory insertions are now thought to proceed by migration of the X group. An important consequence of this pathway is that the relative positions of the other groups on the migrating atom are left unchanged, so the stereochemistry at the X group is preserved.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


