
Structure of clusters
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص564-565
الجزء والصفحة:
ص564-565
 2025-10-06
2025-10-06
 261
261
Structure of clusters
Key points: A cluster includes all compounds with metal-metal bonds that form triangular or larger cyclic structures.
A rigorous definition of metal clusters restricts them to molecular complexes with metal-metal bonds that form triangular or larger cyclic structures. This definition excludes linear MM compounds and cage compounds, in which several metal atoms are held together exclusively by ligand bridges. However, this rigorous definition is normally relaxed, and we shall consider any MM bonded system as a cluster.

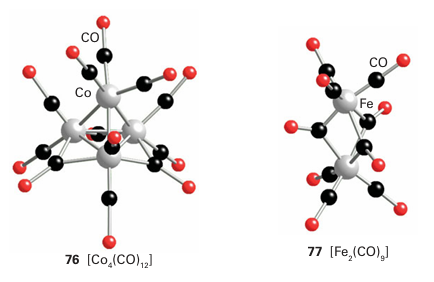
The distinction between cage and cluster compounds can seem arbitrary as the presence of bridging ligands in a cluster such as (76) raises the possibility that the atoms are held together by M L M interactions rather than MM bonds. Bond lengths are of some help in resolving this issue. If the MM distance is much greater than twice the metallic radius, then it is reasonable to conclude that the MM bond is either very weak or absent. However, if the metal atoms are within a reasonable bonding distance, the proportion of the bonding that is attribut able to direct MM interaction is ambiguous. For example, there has been much debate about the extent of Fe-Fe bonding in [Fe2 (CO)9] (77). Metal-metal bond strengths in metal complexes cannot be determined with great precision, but a variety of pieces of evidence—such as the stability of compounds and MM force constants—indicate that there is an increase in M-M bond strengths down a group in the d block. This trend contrasts with that in the p block, where element-element bonds are usually weaker for the heavier members of a group. As a consequence of this trend, metal-metal bonded systems are most numerous for the 4d- and 5d-series metals.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


