
Oxidation and reduction of carbonyls
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص555-556
الجزء والصفحة:
ص555-556
 2025-10-02
2025-10-02
 320
320
Oxidation and reduction of carbonyls
Key points: Most metal carbonyls can be reduced to metal carbonylates; some metal carbonyls disproportionate in the presence of a strongly basic ligand, producing the ligated cation and a carbonylate anion; metal carbonyls are susceptible to oxidation by air; metal-metal bonds undergo oxidative cleavage.

Most neutral metal carbonyl complexes can be reduced to an anionic form known as a met al carbonylate. In monometallic carbonyls, two-electron reduction is generally accompanied by loss of the two-electron donor CO ligand, thus preserving the electron count at 18:
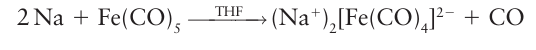
The metal carbonylate contains Fe with oxidation number 2, and it is rapidly oxidized by air. That much of the negative charge is delocalized over the CO ligands is confirmed by the observation of a low CO stretching band in the IR spectrum at about 1730 cm1. Polynuclear carbonyls, which obey the 18-electron rule through the formation of MM bonds, are generally cleaved by strong reducing agents. The 18-electron rule is obeyed in the product and a mononegative mononuclear carbonylate results:
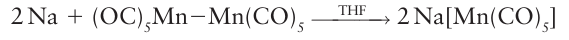
Some metal carbonyls disproportionate in the presence of a strongly basic ligand, producing the ligated cation and a carbonylate. Much of the driving force for this reaction is the stability of the metal cation when it is surrounded by strongly basic ligands. Octacarbonyldicobalt (0) is highly susceptible to this type of reaction when exposed to a good Lewis base such as pyridine (py):
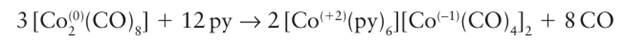
It is also possible for the CO ligand to be oxidized in the presence of the strongly basic ligand OH, the net outcome being the reduction of a metal centre:
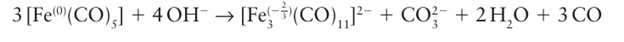
Carbonyl compounds that have only 17 electrons are particularly prone to reduction to give 18-electron carbonylates. Metal carbonyls are susceptible to oxidation by air. Although uncontrolled oxidation produces the metal oxide and CO or CO2, of more interest in organometallic chemistry are the controlled reactions that give rise to organometallic halides. One of the simplest of these is the oxidative cleavage of an MM bond:
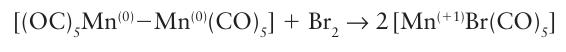
In keeping with the loss of electron density from the metal when a halogen atom is attached, the CO stretching frequencies of the product are significantly higher than those of [Mn2(CO)10].
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


