
Synthesis of homoleptic carbonyls
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص554-555
الجزء والصفحة:
ص554-555
 2025-10-02
2025-10-02
 346
346
Synthesis of homoleptic carbonyls
Key points: Some metal carbonyls are formed by direct reaction, but of those that can be formed in this way most require high pressures and temperatures; metal carbonyls are commonly formed by reductive carbonylation.
The two principal methods for the synthesis of monometallic metal carbonyls are direct combination of carbon monoxide with a finely divided metal and the reduction of a metal salt in the presence of carbon monoxide under pressure. Many polymetallic carbonyls are synthesized from monometallic carbonyls. In 1890 Mond, Langer, and Quinke discovered that the direct combination of nickel and carbon monoxide produced tetracarbonyl nickel (0), Ni (CO)4, a reaction that is used in the Mond process for purifying nickel (Box 22.1):
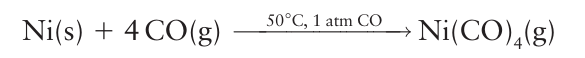
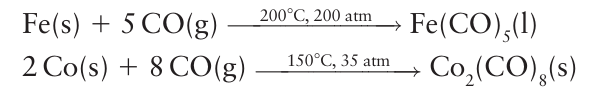
Tetracarbonyl nickel (0) is in fact the metal carbonyl that is most readily synthesized in this way, with other metal carbonyls, such as Fe (CO)5, being formed more slowly. They are therefore synthesized at high pressures and temperatures (Fig. 22.11):
Direct reaction is impractical for most of the remaining d metals, and reductive carbonylation, the reduction of a salt or metal complex in the presence of CO, is normally employed instead. Reducing agents vary from active metals such as aluminium and sodium, to alkyl aluminium compounds, H2, and CO itself:
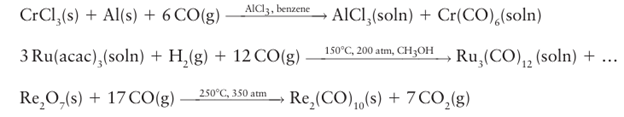
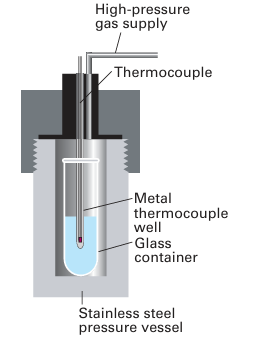
Figure 22.11 A high-pressure reaction vessel. The reaction mixture is in a glass container.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


