
Dinitrogen and nitrogen monoxide
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص552-553
الجزء والصفحة:
ص552-553
 2025-10-01
2025-10-01
 337
337
Dinitrogen and nitrogen monoxide
Key points: The bonding of dinitrogen to a metal atom is weak but contains both a σ-donating and a π-accepting component; nitrogen monoxide can bind to a metal in two different ways—either bent or straight. Neither dinitrogen, N2, nor nitrogen monoxide, NO, is strictly an organometallic ligand, although they are sometimes found in organometallic compounds. Dinitrogen is a much sought-after ligand as complexes can potentially take part in a catalytic reduction of nitro gen to more useful species. Dinitrogen can bind to metals in a number of different ways. The majority of complexes have a terminal monohapto link, η1-N2, in which the bonding can be considered to be like that of the isoelectronic CO ligand (63). Dinitrogen is both a weaker σ donor and a weaker π acceptor than CO, and hence is bound less strongly; in fact only good π-donor metal atoms bind N2. Like CO, the N2 ligand has a distinctive IR stretching band lying in the range 2150-1900 cm-1. A dinitrogen molecule can participate in two bonding interactions and bridge two metal atoms (64). If the backdonation to the nitrogen is extensive in this kind of complex, it can formally be considered to have been reduced to a hydrazine (65). Occasionally, dinitrogen ligands are found bound in a dihapto (η2) side-on fashion (66). In these complexes the ligand is best considered analogous to an η2-alkyne. The side-on bonding mode seems to be particularly common in complexes of the f metals (Chapter 23). Nitrogen monoxide (nitric oxide) is a radical with 11 valence electrons. When bound, NO is referred to as the nitrosyl ligand and can bond in one of two modes to d-metal atoms, in either a bent or a linear fashion. In the linear arrangement (67), the ligand is considered to be the NO cation. The NO cation is isoelectronic with CO, and the bonding can be considered in a similar fashion (a two-electron σ donor, with strong π-acceptor ability).
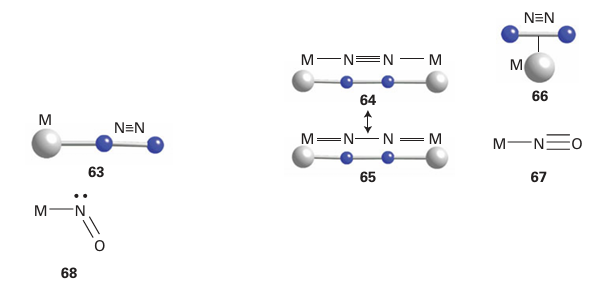
In the bent arrangement (68), NO is considered to behave as NO, again donating two electrons. In many complexes, NO can change its coordination mode; in effect, moving from the linear to the bent mode reduces the number of electrons on the metal by 2.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


