
Benzene and other arenes
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص548-549
الجزء والصفحة:
ص548-549
 2025-10-01
2025-10-01
 404
404
Benzene and other arenes
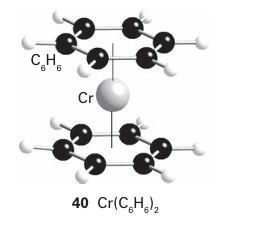
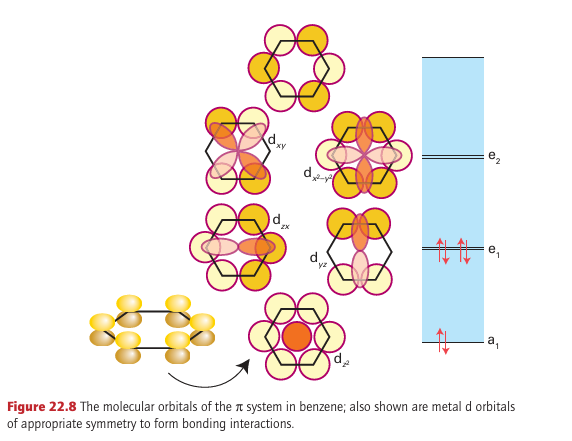
Key point: A consideration of the MOs of benzene leads to a picture of bonding of benzene to a metal atom that includes a significant δ back bonding interaction. If benzene is considered to have three localized double bonds, each double bond can behave as a ligand and the molecule could behave as a tridentate η6-ligand. A compound such as bis(η6-benzene) chromium (40) could then be considered to be made up of six coordinated double bonds, each donating two electrons, bound to a d6 metal atom, giving a total of 18 valence electrons for the octahedral complex. Bis(η6-benzene) chromium does exist, and is remarkably stable: it can be handled in air and sublimes with no decomposition. Although this description of the bonding is a first step towards understanding its structure, the true picture needs a deeper consideration of the molecular orbitals involved. In the molecular orbital picture of the π bonding in benzene there are three bonding and three antibonding orbitals. If we consider a single benzene molecule bonding to a single metal, and consider only the d orbitals, the strongest interaction is a σ interaction between the most strongly bonding a1 benzene MO and the dz2 orbital of the metal atom; π bonds are possible between the two other bonding benzene MOs and the dzx and dyz orbitals. Back bonding from the metal atom to the benzene is possible as a δ interaction between the dx2-y2 and dxy orbitals and the empty antibonding e2 orbitals of benzene (Fig. 22.8). η6-Arenes are considered to be neutral ligands that donate six electrons and are normally considered to take up three coordination sites at a metal.
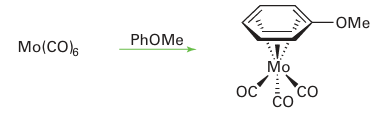
Hexahapto (η6) arene complexes are very easy to make, often simply by dissolving a compound that has three replaceable ligands in the arene and refluxing the solution:
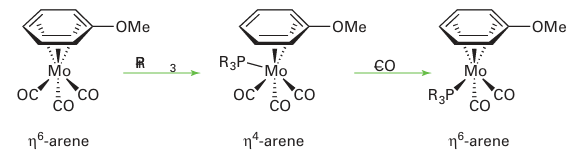
One commonly invoked reaction intermediate of η6-arene complexes is a ‘slipping’ to an η4 complex, which donates only four electrons to the metal, and therefore allows a substitution reaction to proceed without an initial ligand loss:
η2-Arenes are also known and are analogous to η2-alkenes; they have an important role in the activation of arenes by metal complexes.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


