
Phosphines
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص 542-543
الجزء والصفحة:
ص 542-543
 2025-09-30
2025-09-30
 347
347
Phosphines
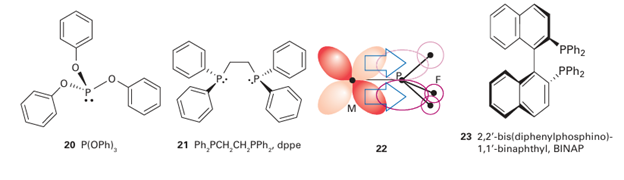
Key point: Phosphines bond to metals by a combination of σ donation from the P atom and π back bonding from the metal atom. Although phosphine complexes are not organometallic because the ligands do not bond to the metal atom through a carbon atom, they are best discussed here as their bonding has many similarities to that of carbon monoxide. Phosphine, PH3 (formally, phosphane), is a reactive, noxious, poisonous, and flammable gas (Section 15.10). Like ammonia, phosphine can behave as a Lewis base and use its lone pair to donate electron density to a Lewis acid, and so act as a ligand. However, given the problems associated with handling phosphine, it is rarely used as a ligand. Substituted phosphines, on the other hand, such as trialkylphosphines (for example, PMe3 , PEt3 ), or triarylphosphines (for example, PPh3 , 10), or trialkyl- or triarylphosphites (for example, P(OMe)3 , P(OPh)3 , 20) and a whole host of bridged multidentate di- and triphosphines (for example, Ph2PCH2CH2 PPh2=dppe, 21) are easy to handle (indeed some are air-stable odourless solids with no appreciable toxicity) and are widely used as ligands; all are colloquially referred to as ‘phosphines’. Phosphines have a lone pair on the P atom that is appreciably basic and nucleophilic, and can serve as a σ donor. Phosphines also have empty orbitals on the P atom that can overlap with filled d orbitals on 3d-metal ions and behave as π acceptors (22). The bond ing of phosphines to a d-metal atom, made up of a σ bond from the ligand to the metal and a π bond from the metal back to the ligand, is completely analogous to the bonding of CO to a d-metal atom. Quite which orbitals on the P atom behave as π acceptors has been the subject of considerable debate in the past with some groups claiming a role for unoccupied 3d orbitals on P, and some claiming a role for the P R σ* orbitals; current consensus favours the σ* orbitals. In any case, each phosphine provides an additional two electrons to the valence electron count. As we have remarked, a huge variety of phosphines are both possible and widely available, including chiral systems such as 2,2-bis(diphenylphosphino)-1,1-binaphthyl (BINAP, 23), in which steric constraints result in compounds that can be resolved into diastereomers. Generally there are two properties of phosphine ligands that are considered important in discussions of the reactivity of their complexes: their steric bulk and their electron donating (and accepting) ability. We described in Section 21.6 how the steric bulk of phosphines can be expressed in terms of the notional cone occupied by the bonded ligand, and Table 22.4 lists some of the derived cone angles. The bonding of phosphines to d-metal atoms is, as we have seen, a composite of σ bonding from the ligand to the metal atom, and π backbonding from the metal atom to the ligand. The σ-donating ability and π-acceptor ability of phosphines are inversely
correlated in the sense that electron-rich phosphines, such as PMe3, are good σ donors and poor π acceptors, whereas electron-poor phosphines, such as PF3, are poor σ donors and good π acceptors.Thus Lewis basicity can normally be used as a single scale to indicate their donor/acceptor ability. The generally accepted order of basicity of phosphines is:
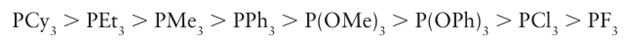
and is easily understood in terms of the electronegativity of the substituents on the P atom. The basicity of a phosphine is not simply related to the strength of the M P bond in a complex, for instance an electron-poor metal atom forms a stronger bond with an electron-rich (basic) phosphine, whereas an electron-rich metal atom will form a stronger bond with an electron-poor phosphine. If there are carbonyl ligands present in a metal phosphine complex, then the carbonyl stretching frequency can be used to assess the basicity of the phosphine ligand: this method allows us to conclude that PF3 is a π acceptor comparable to CO. The vast range of phosphines that are commonly used in organometallic chemistry is a testament to their versatility as ligands: judicious choice allows control over both steric and electronic properties of the metal atom in a complex. Phosphorus-31 (which occurs in 100 per cent natural abundance) is easy to observe by NMR and both the 31P chemical shift and the coupling constant to the metal atom (where appropriate) give consider able insight into the bonding and reactivity of a complex. Like carbonyls, phosphines can bridge either two or three metal atoms, providing additional variety in bonding modes.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


