
18-Electron compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص535-536
الجزء والصفحة:
ص535-536
 2025-09-30
2025-09-30
 340
340
18-Electron compounds
Key points: Six-bonding interactions are possible in an octahedral complex and, when π-acceptor ligands are present, bonding combinations can be made with the three orbitals of the t2g set, leading to nine bonding MOs, and space for a total of 18 electrons. In the 1920s, N.V. Sidgwick recognized that the metal atom in a simple metal carbonyl, such as Ni(CO)4 , has the same valence electron count (18) as the noble gas that terminates the long period to which the metal belongs. Sidgwick coined the term ‘inert gas rule’ for this indication of stability, but it is now usually referred to as the 18-electron rule.1 It becomes readily apparent, however, that the 18-electron rule is not as uniformly obeyed for d-block organometallic compounds as the octet rule is obeyed for compounds of Period 2 elements, and we need to look more closely at the bonding to establish the reasons for the stability of both the compounds that have the 18-electron configurations and those that do not. Figure 22.1 shows the energy levels that arise when a strong-field ligand such as carbon monoxide bonds to a d-metal atom (Section 20.2). Carbon monoxide is a strong-field ligand, even though it is a poor Ϭ donor, because it can use its empty π* orbitals to act as a good π acceptor. In this picture of the bonding, the t2g orbitals of the metal atom are no longer nonbonding, as they would be in the absence of π interactions, but are bonding. The energy level diagram shows six bonding MOs that result from the ligand metal interactions, and three bonding MOs that result from π interactions. Thus up to 18 electrons can be accommodated in the nine bonding MOs. Compounds that have this configuration are remarkably stable, for instance the 18-electron Cr(CO)6 is a colourless air-stable compound. An indication of the size of the HOMO-LUMO gap (∆O ) can be gained from a consideration of its lack of colour, which results from a lack of any electronic transitions in the visible region of the spectrum, that is ∆O is so large that such transitions are shifted to the UV. The only way to accommodate more than 18 valence electrons in an octahedral com plex with strong-field ligands is to use an antibonding orbital. As a result, such complexes are unstable, being particularly prone to electron loss and acting as reducing agents. Com pounds with fewer than 18 electrons will not necessarily be very unstable, but such complexes will find it energetically favourable to acquire extra electrons by reaction and so populate their bonding MOs fully. As we shall see later, compounds with fewer than 18 electrons often occur as intermediates in reaction pathways.
The bonding characteristic of the carbonyl ligand is replicated with other ligands, which are often poor donors but good π acceptors. Hence, octahedral organometallic compounds are most stable when they have a total of 18 valence electrons around their central metal ion. Similar arguments can be used to rationalize the stability of the 18-electron configuration for other geometries, such as tetrahedral and trigonal bipyramidal, although in practice relatively few tetrahedral organometallic compounds are known. The steric require ments of most ligands normally preclude coordination numbers of greater than six for d-metal organometallic compounds.
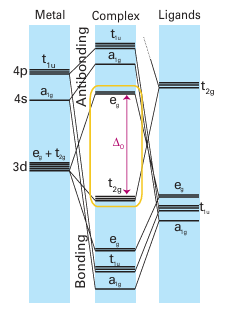
Figure 22.1 The energy levels of the d orbitals of an octahedral complex with strong-field ligands.
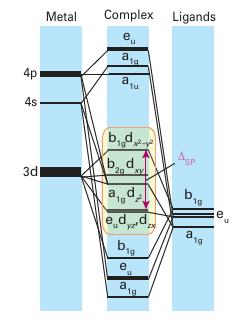
Figure 22.2 The energy levels of the molecular orbitals of a square-planar complex with strong-field ligands. The eight lowest MOs correspond to bonding interactions, with the higher MOs corresponding to antibonding interactions; the MOs are labelled with the d orbitals from which they are derived.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


