
Temperature and pressure dependence
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص515-516
الجزء والصفحة:
ص515-516
 2025-09-29
2025-09-29
 318
318
Temperature and pressure dependence
Key point: Negative volumes and entropies of activation support the view that the rate-determining step of square-planar Pt (II) complexes is associative. Another clue to the nature of the transition state comes from the entropies and volumes of activation for reactions of Pt (II) and Au (III) complexes (Table 21.5). The entropy of activation is obtained from the temperature dependence of the rate constant, and indicates the change in disorder (of reactants and solvent) when the transition state forms. Likewise, the volume of activation, which is obtained (with considerable difficulty) from the pressure dependence of the rate constant, is the change in volume that occurs on formation of the transition state. 1Note that the stereochemistry is quite different from that of p-block central atoms, such as Si (IV) and P(V), where the leaving group departs from the more crowded axial position.
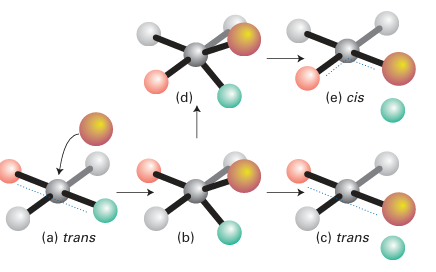
Figure 21.9 The stereochemistry of substitution in a square-planar complex. The normal path (resulting in retention) is from (a) to (c). However, if intermediate (b) is sufficiently long-lived, it can undergo pseudo rotation to (d), which leads to isomer (e).
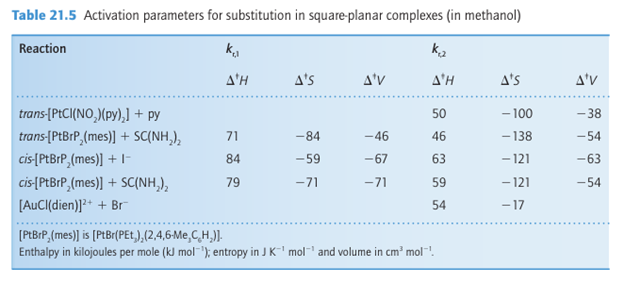
The limiting cases for the volume of activation in ligand substitution reactions correspond to the increase in molar volume of the outgoing ligand (for a dissociative reaction) and the decrease in molar volume of the incoming ligand (for an associative reaction). The two striking aspects of the data in the table are the consistently strongly negative values of both quantities. The simplest explanation of the decrease in disorder and the decrease in volume is that the entering ligand is being incorporated into the transition state without release of the leaving group. That is, we can conclude that the rate-determining step is associative.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


