
Stereochemistry
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
515
الجزء والصفحة:
515
 2025-09-29
2025-09-29
 322
322
Stereochemistry
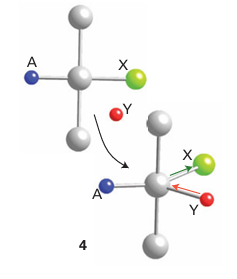
Key point: Substitution of a square-planar complex preserves the original geometry, which suggests a trigonal-pyramidal transition state. Further insight into the nature of the transition state is obtained from the observation that substitution of a square-planar complex preserves the original geometry. That is, a cis complex gives a cis product and a trans complex gives a trans product. This behaviour is explained by the formation of an approximately trigonal-bipyramidal transition state with the entering, leaving, and trans groups in the trigonal plane (4).1 Trigonal-bipyramidal in termediates of this type account for the relatively small influence that the two cis spectator ligands have on the rate of substitution, as their bonding orbitals will be largely unaffected by the course of the reaction. The steric course of the reaction is shown in Fig. 21.9. We can expect a cis ligand to exchange places with the T ligand in the trigonal plane only if the intermediate lives long enough to be stereomobile. That is, it must be a long-lived associative (A) intermediate, with release of the ligand from the five-coordinate intermediate being the rate-determining step.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


