
The nucleophilicity of the entering group
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص513-514
الجزء والصفحة:
ص513-514
 2025-09-29
2025-09-29
 348
348
The nucleophilicity of the entering group
Key points: The nucleophilicity of an entering group is expressed in terms of the nucleophilicity parameter defined in terms of the substitution reactions of a specific square-planar platinum complex; the sensitivity of other platinum complexes to changes in the entering group is expressed in terms of the nucleophilic discrimination factor.
We start by considering the variation of the rate of the reaction as the entering group Y is varied. The reactivity of Y (for instance, I in the reaction above) can be expressed in terms of a nucleophilicity parameter, nPt:
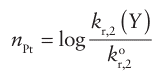
Where kr,2 (Y) is the second-order rate constant for the reaction
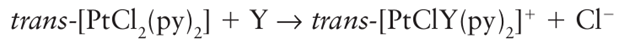
And kr,2o is the rate constant for the same reaction with the reference nucleophile methanol. The entering group is highly nucleophilic, or has a high nucleophilicity, if nPt is large. Table 21.2 gives some values of nPt. One striking feature of the data is that, although the entering groups in the table are all quite simple, the rate constants span nearly nine orders of magnitude. Another feature is that the nucleophilicity of the entering group towards Pt appears to correlate with soft Lewis basicity (Section 4.12), with Cl<I-, O<S, and NH3<PR3.
The nucleophilicity parameter is defined in terms of the reaction rates of a specific platinum complex. When the complex itself is varied we find that the reaction rates show a range of different sensitivities towards changes in the entering group. To express this range of sensitivities we rearrange eqn 21.4 into
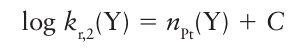
Where C=log kr,2o. Now consider the analogous substitution reactions for the general complex [PtL3 X]:
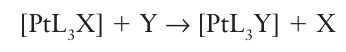
The relative rates of these reactions can be expressed in terms of the same nucleophilicity parameter Pt provided we replace eqn 21.5 by
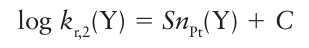
The parameter S, which characterizes the sensitivity of the rate constant to the nucleophilic ity parameter, is called the nucleophilic discrimination factor. We see that the straight line obtained by plotting log kr,2 (Y) against nPt for reactions of Y with trans- [PtCl2(PEt3 )2], the red circles in Fig. 21.8, is steeper than that for reactions with cis-[PtCl2(en)], the blue squares in Fig. 21.8. Hence, S is larger for the former reaction, which indicates that the rate of the reaction is more sensitive to changes in the nucleophilicity of the entering group. Some values of S are given in Table 21.3. Note that S is close to 1 in all cases, so all the complexes are quite sensitive to nPt. This sensitivity is what we expect for associatively activated reactions. Another feature to note is that larger values of S are found for complexes of platinum with softer base ligands.

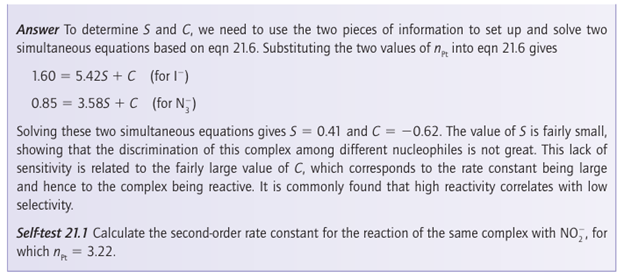
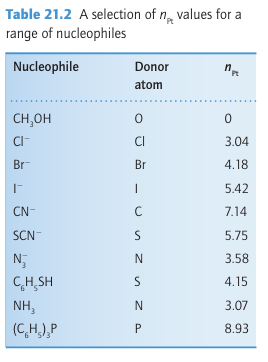
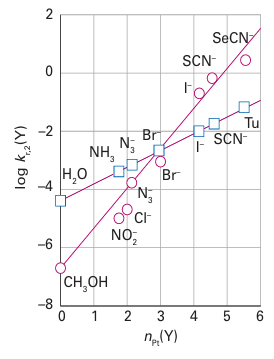
Figure 21.8 The slope of the straight line obtained by plotting log kr,2 (Y) against the nucleophilicity parameter nPt (Y) for a series of ligands is a measure of the responsiveness of the complex to the nucleophilicity of the entering group (data from U. Belluso, L. Cattaini, F. Basolo, R.G. Pearson, and A. Turco; J. Am. Chem. Soc., 1965, 87, 241).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


