
The rate-determining step
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص511-512
الجزء والصفحة:
ص511-512
 2025-09-29
2025-09-29
 324
324
The rate-determining step
Key point: The rate-determining step is classified as associative or dissociative according to the dependence of its rate on the identity of the entering group. Now we consider the rate-determining step of a reaction and the details of its formation. The step is called associative and denoted a if its rate depends strongly on the identity of the incoming group. Examples are found among reactions of the d8 square-planar complexes of Pt (II), Pd (II), and Au (III), including
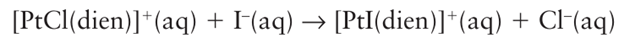
where dien is diethylenetriamine (NH2 CH2CH2 NHCH2 CH2 NH2). It is found, for instance, that use of I instead of Br increases the rate constant by an order of magnitude. Experi mental observations on the substitution reactions of square-planar complexes support the view that the rate-determining step is associative.
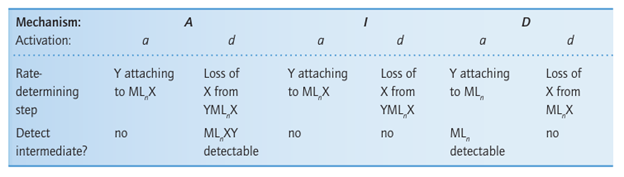
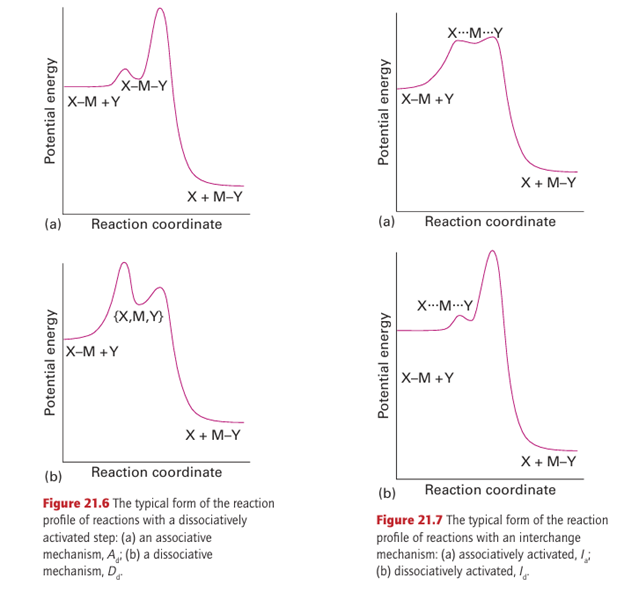
The strong dependence of the rate-determining step on entering group Y indicates that the transition state must involve significant bonding to Y. A reaction with an associative mechanism (A) will be associatively activated (a) if the attachment of Y to the initial react ant MLnX is the rate-determining step; such a reaction is designated Aa, and in this case the intermediate MLnXY would not be detected. A reaction with a dissociative mechanism (D) is associatively activated (a) if the attachment of Y to the intermediate MLn is the rate-determining step; such a reaction is designated Da. Figure 21.5 shows the reaction profiles for associatively activated A and D mechanisms. For the reactions to proceed, it is necessary to have established a population of an encounter complex X-M, Y in a pre-equilibrium step.
The rate-determining step is called dissociative and denoted d if its rate is largely independent of the identity of Y. This category includes some of the classic examples of ligand substitution in octahedral d-metal complexes, including
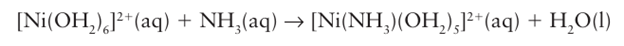
It is found that replacement of NH3 by pyridine in this reaction changes the rate by at most a few per cent. The weak dependence on Y of a dissociatively activated process indicates that the rate of formation of the transition state is determined largely by the rate at which the bond to the leaving group X can break. A reaction with an associative mechanism (A) will be dissociatively activated (d) provided the loss of X from the intermediate YMLn X is the rate determining step; such a reaction is designated Ad. A reaction with a dissociative mechanism (D) is dissociatively activated (d) if the initial loss of X from the reactant MLn X is the rate-determining step, such a reaction is designated Dd. In this case, the intermediate MLn would not be detected. Figure 21.6 shows the reaction profiles for dissociatively activated A and D mechanisms.
A reaction that has an interchange mechanism (I) can be either associatively or dissociatively activated, and is designated either Ia or Id, respectively. In an Ia mechanism, the rate of reaction depends on the rate at which the M…Y bond forms, whereas in an Id reaction the rate of reaction depends on the rate at which the M…X bond breaks (Fig. 21.7). The distinction between these possibilities may be summarized as follows, where MLnX denotes the initial complex:
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


