
Luminescence
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص501-502
الجزء والصفحة:
ص501-502
 2025-09-28
2025-09-28
 311
311
Luminescence
Key points: A luminescent complex is one that re-emits radiation after it has been electronically excited. Fluorescence occurs when there is no change in multiplicity, whereas phosphorescence occurs when an excited state undergoes intersystem crossing to a state of different multiplicity and then undergoes radiative decay. A complex is luminescent if it emits radiation after it has been electronically excited by the absorption of radiation. Luminescence competes with nonradiative decay by thermal degradation of energy to the surroundings. Relatively fast radiative decay is not especially common at room temperature for d-metal complexes, so strongly luminescent systems are comparatively rare. Nevertheless, they do occur, and we can distinguish two types of process. Traditionally, rapidly decaying luminescence was called ‘fluorescence’ and luminescence that persists after the exciting illumination is extinguished was called ‘phosphor essence’. However, because the lifetime criterion is not reliable, the modern definitions of the two kinds of luminescence are based on the distinctive mechanisms of the processes. Fluorescence is radiative decay from an excited state of the same multiplicity as the ground state. The transition is spin-allowed and is fast; fluorescence half-lives are a matter of nanoseconds. Phosphorescence is radiative decay from a state of different multiplicity from the ground state. It is a spin-forbidden process, and hence is often slow. The initial excitation of a phosphorescent complex usually populates a state by a spin allowed transition, so the mechanism of phosphorescence involves intersystem crossing, the nonradiative conversion of the initial excited state into another excited state of different multiplicity. This second state acts as an energy reservoir because radiative decay to the ground state is spin-forbidden. However, just as spin orbit coupling allows the inter system crossing to occur, it also breaks down the spin selection rule, so the radiative decay can occur. Radiative decay back to the ground state is slow, so a phosphorescent state of a d-metal complex may survive for microseconds or even longer. An important example of phosphorescence is provided by ruby, which consists of a low concentration of Cr3 ions in place of Al3 in alumina. Each Cr3 ion is surrounded octa hedrally by six O2 ions and the initial excitations are the spin-allowed processes
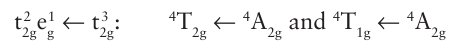
These absorptions occur in the green and violet regions of the spectrum and are responsible for the red colour of the gem (Fig. 20.35). Intersystem crossing to a 2E term of the t2g3 configuration occurs in a few picoseconds or less, and red 627 nm phosphorescence occurs as this doublet decays back into the quartet ground state. This red emission adds to the red perceived by the subtraction of green and violet light from white light, and adds lustre to the gem’s appearance. This effect was utilised in the first laser to be constructed (in 1960). A similar 2E → 4A phosphorescence can be observed from a number of Cr (III) complexes in solution. The 2E term arises from the t2g 3 configuration, which is the same as the ground state, and thus the strength of the ligand field is not important. Hence the emission is always in the red (and close to the wavelength of ruby emission). If the ligands are rigid, as in [Cr(bpy)3] +3, the 2E term may live for several microseconds in solution. Another interesting example of a phosphorescent state is found in [Ru(bpy)3]2+. The excited singlet term produced by a spin-allowed MLCT transition of this d6 complex under goes intersystem crossing to the lower energy triplet term of the same configuration, t2g 5π*1. Bright orange emission then occurs with a lifetime of about 1 s (Fig. 20.36). The effects of other molecules (quenchers) on the lifetime of the emission may be used to monitor the rate of electron transfer from the excited state.
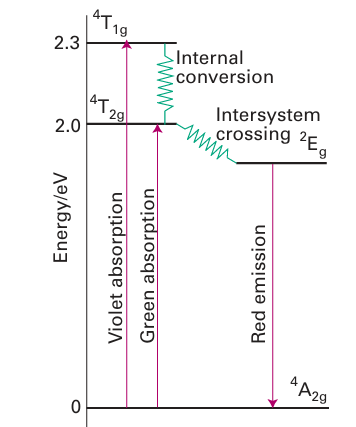
Figure 20.35 The transitions responsible for the absorption and luminescence of Cr3+ ions in ruby.
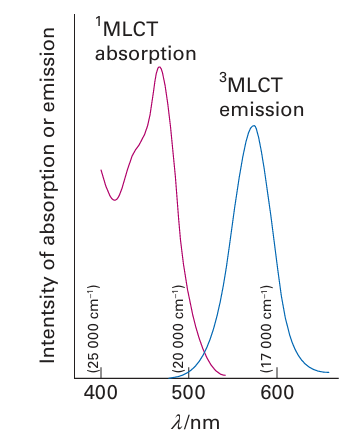
Figure 20.36 The absorption and phosphorescence spectra of [Ru(bpy)3]2+.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


