
Ligand-field transitions
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
493
الجزء والصفحة:
493
 2025-09-28
2025-09-28
 314
314
Ligand-field transitions
Key point: Electron-electron repulsion splits ligand-field transitions into components with different energies. According to the discussion in Section 20.1, we expect the octahedral d3 complex [Cr (NH3)6 ]3 to have the ground-state configuration t2g3. The absorption near 25 000 cm1 can be identified as arising from the excitation t2g2 eg1 ← t2g3 because the corresponding energy (close to 3 eV) is typical of ligand-field splittings in complexes. Before we embark on a Racah-like analysis of the transition, it will be helpful to see qualitatively from the viewpoint of molecular orbital theory why the transition gives rise to two bands. First, note that a dz2 ← dxy transition, which is one way of achieving eg ← t2g, promotes an electron from the xy-plane into the already electron-rich z-direction: that axis is electron-rich because both dyz and dzx are occupied (Fig. 20.24). However, a dz2 ← dzx transition, which is another way of achieving eg ← t2g, merely relocates an electron that is already largely concentrated along the z-axis. In the former case, but not in the latter, there is a dis tinct increase in electron repulsion and, as a result, the two eg ← t2g transitions lie at different energies. There are six possible t2g2 eg1 ← t2g3 transitions, and all resemble one or other of these two cases: three of them fall into one group and the other three fall into the second group.
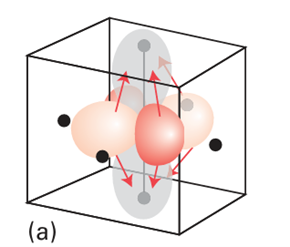
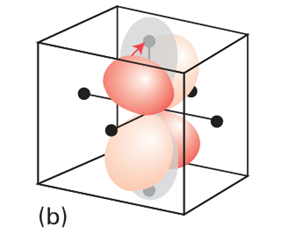
Figure 20.24 The shifts in electron density that accompany the two transitions discussed in the text. There is a considerable relocation of electron density towards the ligands on the z-axis in (a), but a much less substantial relocation in (b).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


