
The classification of microstates
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
490-491
الجزء والصفحة:
490-491
 2025-09-28
2025-09-28
 303
303
The classification of microstates
Key point: The allowed terms of a configuration are found by identifying the values of L and S to which the microstates of an atom can contribute.
The Pauli principle restricts the microstates that can occur in a configuration and consequently affects the terms that can occur. For example, two electrons cannot both have the same spin and be in a d orbital with ml=2. Therefore, the microstate (2+ ,+2 ) is for bidden and so are the values of L and S to which such a microstate might contribute. We shall illustrate how to determine what terms are allowed by considering a d2 configuration, as the outcome will be useful in the discussion of the complexes encountered later in the chapter. An example of a species with a d2 configuration is a Ti+2 ion. We start the analysis by setting up a table of microstates of the d2 configuration (Table 20.6); only the microstates allowed by the Pauli principle have been included. We then use a process of elimination to classify all the microstates. First, we note the largest value of ML, which for a d2 configuration is +4. This state must belong to a term with L=4 (a G term). Table 20.6 shows that the only value of MS that occurs for this term is MS=0, so the G microstates in each of the boxes in the column below (2 ,2 ) must belong to this term.3 We can therefore strike out one microstate from each row in the central column of Table 20.6, which leaves 36 microstates.
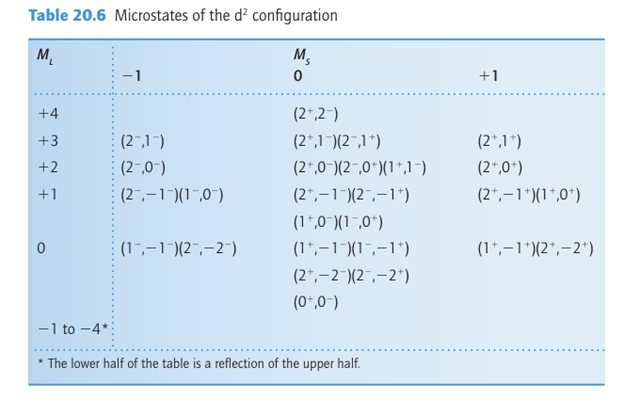
3In fact, it is unlikely that one of the microstates itself will correspond to one of these states: in general, a state is a linear combination of microstates. However, as N linear combinations can be formed from N microstates, each time we cross off one microstate, we are taking one linear combination into account, so the bookkeeping is correct even though the detail may be wrong.
The next largest value is ML=+3, which must stem from L=3 and hence belong to an F term. That row contains one microstate in each column (that is, each box contains one unassigned combination for MS 1, 0, and +1), which signifies S=1 and there-fore a triplet term. Hence the microstates belong to 3F. The same is true for one micro state in each of the rows down to ML=-3, which accounts for a further 3x7=21 microstates. If we strike out one state in each of the 21 boxes, we are left with 15 to be assigned.
There is one unassigned microstate in the row with ML=+2 (which must arise from L=2) and the column under MS=0 (S=0), which must therefore belong to a 1D term. This term has five values of ML, which removes one microstate from each row in the column headed MS 0 down to ML=-2, leaving 10 microstates unassigned. Because these unassigned microstates include one with ML=+1 and MS=+1, nine of these microstates must belong to a 3P term. There now remains only one microstate in the central box of the table, with ML=0 and MS=0. This microstate must be the one and only state of a 1S term (which has L=0 and S=0).
At this point we can conclude that the terms of a 3d2 configuration are 1G, 3F, 1D, 3P, and 1S. These terms account for all 45 permitted states (see table in the margin).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


