
Electronic spectra of atoms
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص487-488
الجزء والصفحة:
ص487-488
 2025-09-28
2025-09-28
 320
320
Electronic spectra of atoms
Key point: Electron-electron repulsions result in multiple absorptions in the electronic spectrum. Figure 20.22 sets the stage for our discussion by showing the electronic absorption spectrum of the d3 complex [Cr (NH3)6]3+ in aqueous solution. The band at lowest energy (longest wavelength) is very weak; later we shall see that it is an example of a ‘spin-forbidden’ transition. Next are two bands with intermediate intensities; these are ‘spin-allowed’ transitions between the t2g and eg orbitals of the complex, which are mainly derived from the
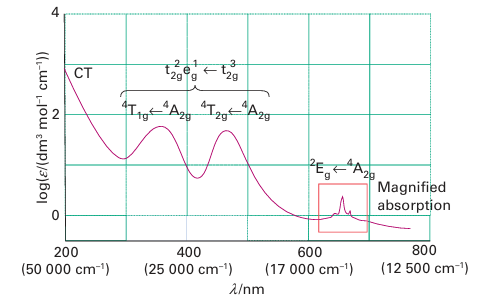
Figure 20.22 The spectrum of the d3complex [Cr (NH3)6]3+, which illustrates the features studied in this section, and the assignments of the transitions as explained in the text.
metal d orbitals. The third feature in the spectrum is an intense charge-transfer band at short wavelength (labelled CT, denoting ‘charge transfer’), of which only the low-energy tail is evident in the illustration. One problem that immediately confronts us is why two absorptions can be ascribed to the apparently single transition t2g2 eg1 ← t2g3. This splitting of a single transition into two bands is in fact an outcome of the electron-electron repulsions mentioned above. To understand how it arises, and to extract the information it contains, we need to consider the spectra of free atoms and ions.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


