
Special properties of fluorine compounds
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص428-429
الجزء والصفحة:
ص428-429
 2025-09-27
2025-09-27
 310
310
Special properties of fluorine compounds
Key points: Fluorine substituents promote volatility, increase the strengths of Lewis and Brønsted acids, and stabilize high oxidation states.
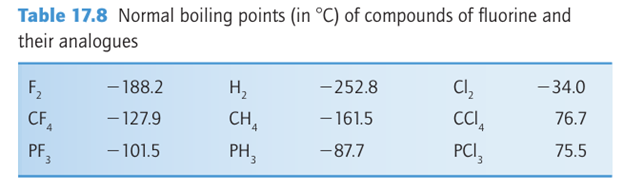
The boiling points in Table 17.8 demonstrate that molecular compounds of F tend to be highly volatile, in some cases even more volatile than the corresponding hydrogen com pounds (compare, for example, PF3, b.p. 101.5C, and PH3 , b.p. 87.7C) and in all cases much more volatile than the Cl analogues. The volatilities of the compounds are a result of variations in the strength of the dispersion interaction (the inter action between instantaneous transient electric dipole moments), which is strongest for highly polarizable molecules. The electrons in the small F atoms are gripped tightly by the nuclei, and consequently F compounds have low polarizabilities and hence weak dispersion interactions. There are some opposite effects on volatility that can be traced to hydrogen bonding. The structure of solid HF is a planar zigzag chain polymer of F H…F units. Although liquid HF has a lower density and viscosity than water, which suggests the absence of an extensive three-dimensional network of H bonds, in the gas phase HF forms H-bonded oligomers, (HF)n with n up to 5 or 6. As with H2O and NH3, the properties of HF, such as a wide liquid range, make it an excellent nonaqueous solvent. Hydrogen fluoride undergoes autoprotolysis:
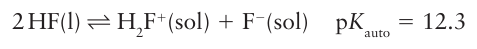
It is a much weaker acid (pKa=3.45 in water) than the other hydrogen halides. Although this difference is sometimes attributed to the formation of an ion pair (H3 O+F-), theoretical considerations show that its poor proton donor properties are a direct result of the very strong H-F bond. Carboxylic acids act as bases in anhydrous HF and are protonated:
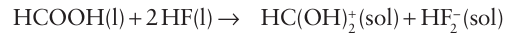
An important characteristic is the ability of an F atom in a com pound to withdraw electrons from the other atoms present and, if the compound is a Brønsted acid, to enhance its acidity. An example of this effect is the increase by three orders of magnitude in the acidity of trifluoromethanesulfonic acid, HOSO2CF3 (pKa 3.0 in nitromethane), over that of methanesulfonic acid, HOSO2CH3 (pKa 6.0 in nitromethane). The presence of F atoms in a molecule also results—for the same reason—in an enhanced Lewis acidity. For example, we saw in Sections 4.15 and 15.11b that SbF5 is one of the strongest Lewis acids of its type and much stronger than SbCl5. Some examples of high oxidation state compounds of F are IF7, PtF6, BiF5, KAgF4, UF6, and ReF7. Rhenium (VII) heptafluoride is the only example of a thermally stable metal heptafluoride and uranium (VI) hexafluoride is important in the separation of U isotopes in the preprocessing of nuclear fuels. All these com pounds are examples of the highest oxidation state attainable for these elements, the rare oxidation state Ag (III) being perhaps the most notable. Another example is the stability of PbF4, compared to all other Pb(IV) halides. A related phenomenon is the tendency of fluorine to disfavour low oxidation states. Thus, solid copper(I) fluoride, CuF, is un stable but CuCl, CuBr, and CuI are stable with respect to disproportionation. Similar trends were discussed in Section 3.11 in terms of a simple ionic model in which the small size of the F ion in combination with a small, highly charged cation results in a high lattice enthalpy. As a result, there is a thermodynamic tendency for CuF to disproportionate and form copper metal and CuF2 (because Cu+2 is doubly charged and its ionic radius is smaller than that of Cu+2 and it has a greater lattice enthalpy). Compounds that accept F-ions are Lewis acids and com pounds that donate F- are Lewis bases:
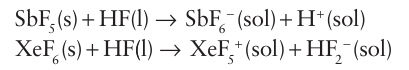
Ionic fluorides dissolve in HF to give highly conducting solutions. The fact that chlorides, bromides, and iodides react with HF to give the corresponding fluoride and HX provides a preparative route to anhydrous fluorides:
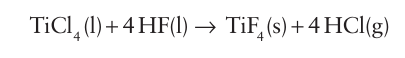
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


