
Pseudohalogens
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
427-728
الجزء والصفحة:
427-728
 2025-09-27
2025-09-27
 295
295
Pseudohalogens
Key points: Pseudohalogens and pseudohalides mimic halogens and halides, respectively; the pseudohalogens exist as dimers and form molecular compounds with nonmetals and ionic compounds with alkali metals.
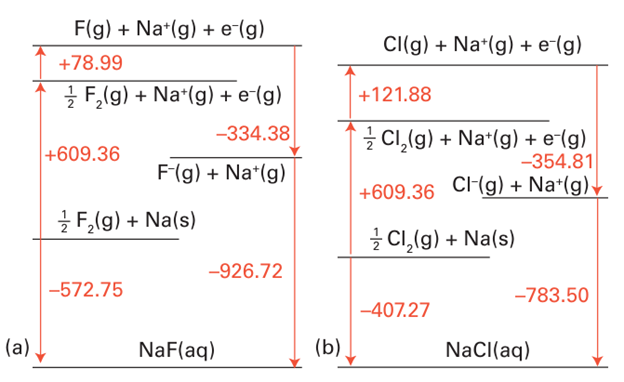
Figure 17.10 Thermochemical cycles for the enthalpy of formation of (a) aqueous sodium fluoride and (b) aqueous sodium chloride. The hydration is much more exothermic for F than for Cl-. All values are in kilojoules per mole (kJ mol1-).
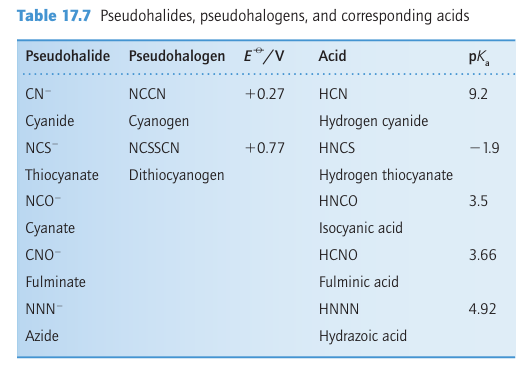
A number of compounds have properties so similar to those of the halogens that they are called pseudohalogens (Table 17.7). For example, like the dihalogens, cyanogen, (CN)2, undergoes thermal and photochemical dissociation in the gas phase; the resulting CN radicals are isolobal with halogen atoms and undergo similar reactions, such as a chain reaction with hydrogen:
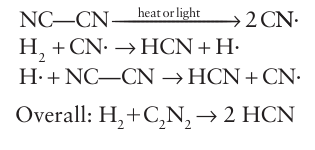
Another similarity is the reduction of a pseudohalogen:
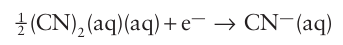
The anion formally derived from a pseudohalogen is called a pseudohalide ion. An example is the cyanide anion, CN. Cova lent pseudohalides similar to the covalent halides of the p-block elements are also common. They are often structurally similar to the corresponding covalent halides (compare (6) and (7)), and undergo similar metathesis reactions. As with all analogies, the concepts of pseudohalogen and pseudohalide have many limitations. For example, pseudo halogen ions are not spherical, so the structures of their ionic compounds often differ: NaCl is fcc but NaCN is similar to CaC2 (Section 11.12). The pseudohalogens are generally less
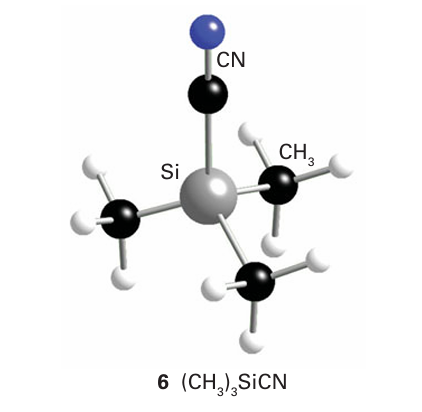
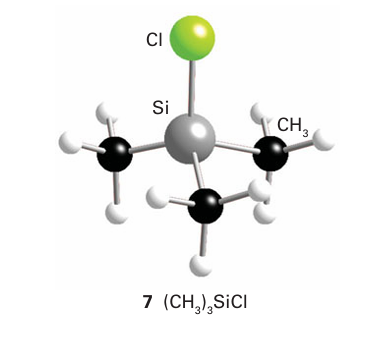
electronegative than the lighter halogens and some pseudo- halides have more versatile donor properties. The thiocyanate ion, SCN, for instance, acts as an ambidentate ligand with a soft base site, S, and a hard base site, N (Section 4.15).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


