
Molecular structure and properties
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص425-427
الجزء والصفحة:
ص425-427
 2025-09-27
2025-09-27
 313
313
Molecular structure and properties
Key points: The F-F bond is weak relative to the Cl-Cl bond; bond strengths decrease down the group from chlorine. Among the most striking physical properties of the halogens are their colours. In the vapour they range from the almost colour less F2, through yellow–green Cl2 and red–brown Br2, to purple I2. The progression of the maximum absorption to longer wavelengths reflects the decrease in the HOMO–LUMO gap on descending the group. In each case, the optical absorption spectrum arises primarily from transitions in which an electron is promoted from the highest filled 2Ϭg and 1 g orbitals into the vacant antibonding 2Ϭu orbital (Fig. 17.5). Except for F2, the analysis of the UV absorption spectra gives precise values for the dihalogen bond dissociation energies (Fig. 17.6). It is found that bond strengths decrease down the group from Cl2. The UV spectrum of F2, however, is a broad
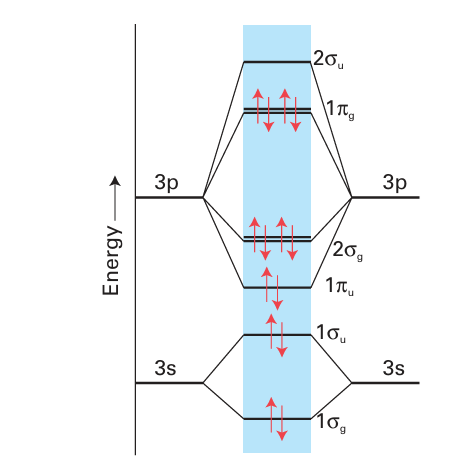
Figure 17.5 Schematic molecular orbital energy level diagram for Cl2 (similarly Br2 and I2). For F2 the order of the u and upper g orbitals is reversed.

continuum that lacks structure because absorption is accompanied by dissociation of the F2 molecule. The lack of discrete absorption bands makes it difficult to estimate the dissociation energy spectroscopically, and thermochemical methods are com plicated by the highly corrosive nature of this reactive halogen. When these problems were solved, the F-F bond enthalpy was found to be less than that of Br2 and thus out of line with the trend in the group. However, the low F-F bond enthalpy is consistent with the low single-bond enthalpies of N-N, O-O, and various combinations of N, F, and O (Fig. 17.7). The simplest explanation (like the explanation of the low electron affinity of fluorine) is that the bond is weakened by the strong repulsions between nonbonding electrons in the small F2 molecule. In molecular orbital terms, the molecule has numerous electrons in strongly antibonding orbitals. Chlorine, bromine, and iodine all crystallize in lattices of the same symmetry (Fig. 17.8), so it is possible to make a detailed comparison of distances between bonded and nonbonded adjacent atoms (Table 17.6). The important conclusion is that non bonded distances do not increase as rapidly as the bond lengths.
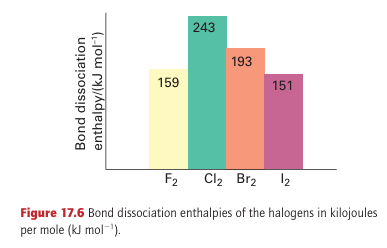
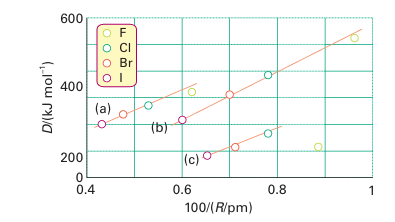
Figure 17.7 Dissociation enthalpies of (a) carbon halogen, (b) hydrogen halogen, and (c) halogen-halogen bonds plotted against the reciprocal of the bond length.
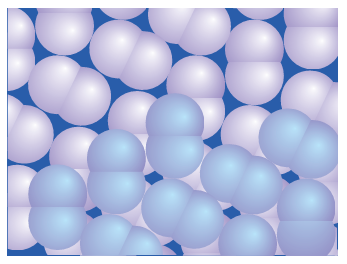
Figure 17.8 Solid chlorine, bromine, and iodine have similar structures. The closest nonbonded interactions are relatively less compressed in Cl2 and Br2 than in I2.
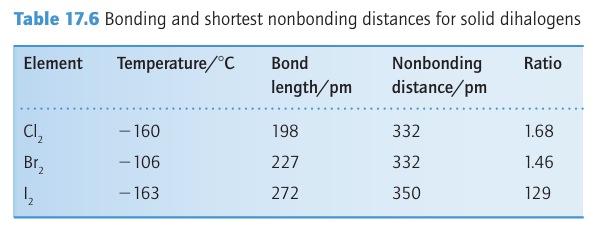
This observation suggests the presence of weak intermolecular bonding interactions that strengthen on going from Cl2 to I2. Solid iodine is a semiconductor and under high pressure exhibits metallic conductivity.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


