
Occurrence, recovery, and uses
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص 424-425
الجزء والصفحة:
ص 424-425
 2025-09-25
2025-09-25
 318
318
Occurrence, recovery, and uses
Key points: Fluorine, chlorine, and bromine are prepared by electrochemical oxidation of halide salts; chlorine is used to oxidize Br and I to the corresponding dihalogen. All the dihalogens (except the radioactive At2) are produced com mercially on a large scale, with chlorine production by far the greatest, followed by fluorine. The principal method of production of the elements is by electrolysis of the halides (Section 5.18). The strongly positive standard potentials EO (F2, F) =2.87 V and EO (Cl2, Cl) 1.36 V indicate that the oxidation of F and Cl ions requires a strong oxidizing agent. Only electro lytic oxidation is commercially feasible. An aqueous electrolyte cannot be used for fluorine production because water is oxidized at a much lower potential (+1.23 V) and any fluorine produced would react rapidly with water. The isolation of elemental fluorine is achieved by electrolysis of a 1:2 mixture of molten KF and HF in a cell like that shown in Fig. 17.3. It is important to keep fluorine and the byproduct, hydrogen, separate because they react violently. Most commercial chlorine is produced by the electrolysis of aqueous sodium chloride solution in a chloralkali cell (Fig. 17.4). The half-reactions are
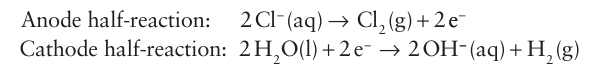
The oxidation of water at the anode is suppressed by using an electrode material that has a higher overpotential for O2 evolution than for Cl2 evolution (Section 5.18). The best anode mate rial seems to be RuO2 (Section 19.8). This process is the basis of
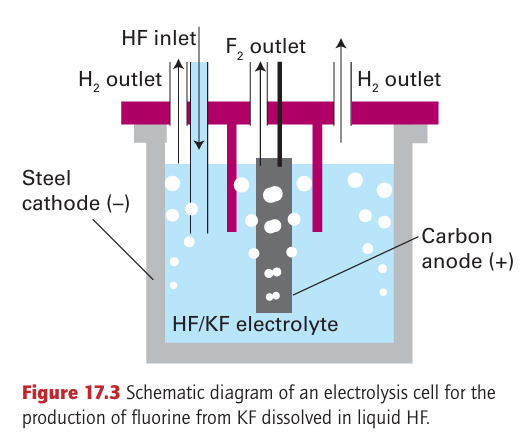
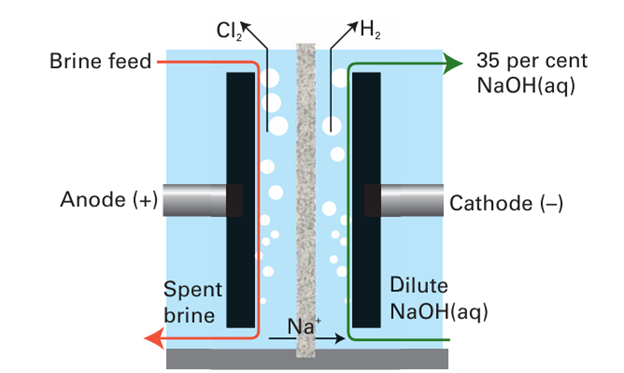
Figure 17.4 Schematic diagram of a chloralkali cell using a cation transport membrane, which has high permeability to Na ions and low permeability to OH- and Cl- ions.
the chloralkali industry, which produces sodium hydroxide on a massive scale (see Box 11.2):
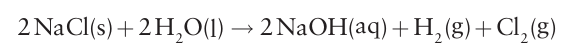
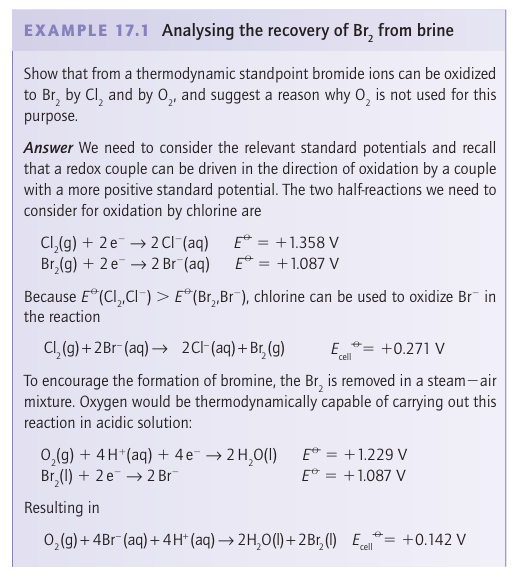
Bromine is obtained by the chemical oxidation of Br ions in seawater. A similar process is used to recover iodine from certain natural brines that are rich in I-. The more strongly oxidizing halogen, chlorine, is used as the oxidizing agent in both processes, and the resulting Br2 and I2 are driven from the solution in a stream of air:
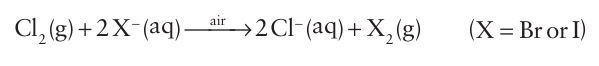

All the Group 17 elements undergo thermal or photochemical dissociation in the gas phase to form radicals. These radicals take part in chain reactions, such as:
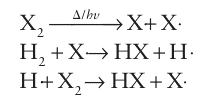
A reaction of this type between chlorine and methane is used in the industrial synthesis of chloroform, CH3 Cl, and dichloro methane, CH2Cl2 . Compounds of F are used throughout industry. Fluorine as F ions is added to some domestic water supplies and toothpaste to prevent tooth decay (Box 17.2). It is used as UF6 in the nuclear power industry for the separation of the isotopes of uranium. Hydrogen fluoride is used to etch glass and as a nonaqueous solvent. Chlorine is widely used in industry to make chlorinated hydrocarbons and in applications in which a strong oxidizing agent is needed, including disinfectants and bleaches. These applications are in decline, however, because some organic Cl compounds are carcinogenic and chlorofluorocarbons (CFCs) are implicated in the destruction of ozone in the stratosphere (Box 17.3). Hydrofluorocarbons (HFCs) are now replacing CFCs in applications such as refrigeration and air conditioning. Organobromine compounds are used in synthetic organic chemistry: the C Br bond is not as strong as the CCl bond and Br can be more readily displaced (and recycled). Iodine is an essential element and iodine deficiency is a cause of goitre, the enlargement of the thyroid gland. For this reason, small amounts of potassium iodide are added to table salt.

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


