
Metal sulfides, selenides, tellurides, and polonides
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص409-410
الجزء والصفحة:
ص409-410
 2025-09-25
2025-09-25
 362
362
Metal sulfides, selenides, tellurides, and polonides
Key point: Monatomic and polyatomic sulfide ions are known as discrete anions and as ligands. Many metals occur naturally as their sulfide ores. The ores are roasted in air to form the oxide or the water-soluble sulfate, from which the metals are extracted. The sulfides can be pre pared in the laboratory or industry by a number of routes; direct combination of the elements, reduction of a sulfate, or precipitation of an insoluble sulfide from solution by addition of H2S:
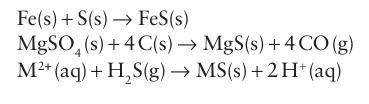
The solubilities of the metal sulfides vary enormously. The Group 1 and 2 sulfides are soluble, whereas the sulfides of the heavy elements of Group 11 and 12 are among the least soluble com pounds known. The wide variation enables selective separation of metals to take place on the basis of the solubilities of the sulfides. The Group 1 sulfides, M2S, adopt the antifluorite structure (Section 3.9). The Group2 elements and some of the f-block elements form monosulfides, MS, with a rock-salt structure. The first-row d-block metals form monosulfides with the NiAs structure, whereas the heavier elements have a greater tendency to covalence and adopt a zinc-blende structure. The d-metals form disulfides that have a layered structure or contain discrete S2-2 ions. These compounds are discussed in Chapter 19. The selenides and tellurides are the most common naturally occurring sources of the elements. Group 1 and 2 selenides, tellurides, and polonides are prepared by direct interaction of the elements in liquid ammonia. They are water-soluble solids that are rapidly oxidized in air to give the elements, with the exception of the polonides, for which they are among the most stable compounds of the element. The selenides and tellurides of Li, Na, and K adopt the antifluorite structure; those of the heavier elements of Group 1 adopt the rock-salt structure. Selenides, tellurides, and polonides of the d metals are also prepared by direct interaction of the elements and are nonstoichiometric.
Two examples are compounds of approximate stoichiometry Ti2Se and Ti3Se.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


