
Thermochemical correlations
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص479-480
الجزء والصفحة:
ص479-480
 2025-09-24
2025-09-24
 312
312
Thermochemical correlations
Key point: The experimental variation in hydration enthalpies reflects a combination of the variation in radii of the ions (the linear trend) and the variation in LFSE (the saw-tooth variation). The concept of ligand-field stabilization energy helps to explain the double-humped variation in the hydration enthalpies of the high-spin octahedral 3d-metal M+2 ions (Fig. 20.7). The nearly linear increase across a period shown by the filled circles represents the increasing strength of the bonding between H2O ligands and the central metal ion as the ionic radii decrease from left to right across the period. The deviation of hydration enthalpies from a straight line reflects the variation in the ligand-field stabilization energies. As Table 20.2 shows, the LFSE increases from d1 to d3, decreases again to d5, then rises to d8. The filled circles in Fig. 20.7 were calculated by subtracting the high-spin LFSE from ∆hyd H by using the spectroscopic values of ∆O in Table 20.1. We see that the LFSE calculated from spectroscopic data accounts for the additional ligand binding energy for the complexes shown in the illustration.
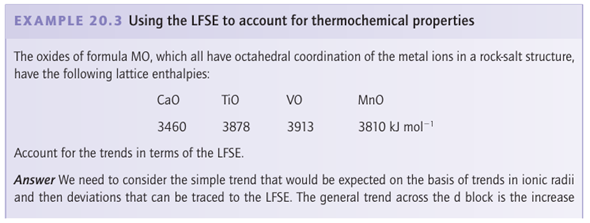

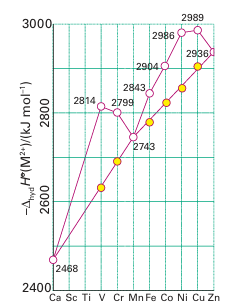
Figure 20.7 The hydration enthalpy of M+2 ions of the first row of the d block. The straight line shows the trend when the ligand-field stabilization energy has been subtracted from the observed values. Note the general trend to greater hydration enthalpy (more exothermic hydration) on crossing the period from left to right.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


