

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Xenon oxygen compounds
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
الجزء والصفحة:
ص444-445
2025-09-22
389
Xenon oxygen compounds
Key point: The xenon oxides are unstable and highly explosive. Xenon oxides are endergonic (ΔfGO>0) and cannot be pre pared by direct interaction of the elements. The oxides and oxo fluorides are prepared by the hydrolysis of xenon fluorides:
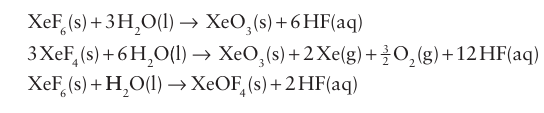
The pyramidal xenon trioxide, XeO3(4) presents a serious hazard because this endergonic compound is highly explosive. It is a very strong oxidizing agent in acidic solution, with E ْ (XeO3, Xe) +=2.10 V. In basic aqueous solution the Xe (VI) oxoanion HXeO4 slowly decomposes in a coupled disproportionation and water oxidation to yield a Xe (VIII) perxenate ion, XeO6-4, and xenon:
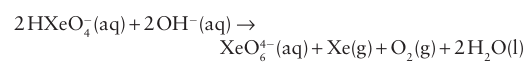
The perxenates of several alkali metal ions have been prepared by treatment of XeO3 with ozone in basic conditions. These compounds are white crystalline solids with octahedral XeO6 4 units (12). They are powerful oxidizing agents in acidic aqueous solution:
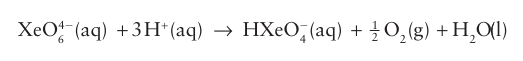
Treating Ba2XeO6 with concentrated sulfuric acid produces the only other known oxide of xenon, XeO4 (5), which is an explosively unstable gas.
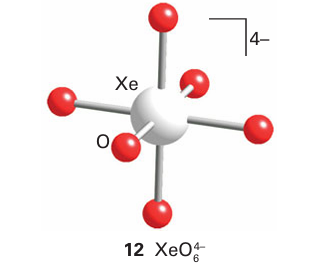
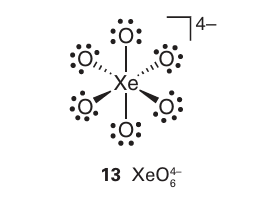
A brief illustration. Thestructure of many xenon compounds can be successfully predicted by using the VSEPR model. The Lewis structure of the perxenate ion is shown in (13). With six electron pairs around the Xe atom, the VSEPR model predicts an octahedral arrangement of bonding electron pairs and an octahedral overall structure.
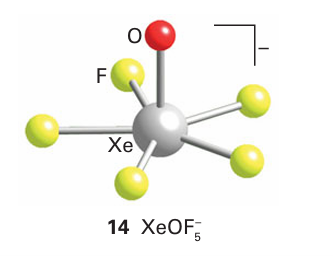
Xenon forms the oxofluorides XeOF2(6), XeO3F2 (7), and XeOF4 (8). When alkali metal fluorides are dissolved in XeOF4, solvated fluoride ions are formed of composition F-.3XeOF4. Attempted removal of XeOF4 from the solvate yields XeOF5 (14), which is a pentagonal pyramid.
A brief illustration. Thestructures of compounds of xenon can be probed using 129Xe-NMR spectroscopy. For example, the 129Xe-NMR spectrum of XeOF4 (8) consists of a quintuplet of peaks. These peaks correspond to the single Xe environment, which is coupled to four equivalent 19F atoms.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















