
Thermodynamic considerations
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص277-278
الجزء والصفحة:
ص277-278
 2025-09-04
2025-09-04
 417
417
Thermodynamic considerations
Key points: In the s and p blocks, strengths of E H bonds decrease down each group. In the d block, strengths of E H bonds increase down each group.
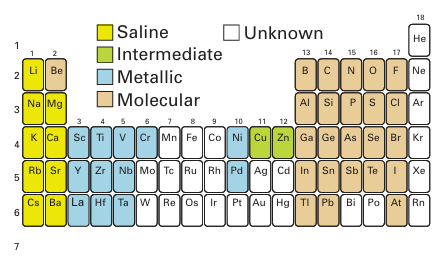
Figure 10.2 Classification of the binary hydrogen compounds of the s-, p-, and d-block elements. Although some d-block elements such as iron and ruthenium do not form binary hydrides they do form metal complexes containing the hydride ligand.The standard Gibbs energies of formation of the hydrogen compounds of s- and p-block elements reveal a regular variation in stability (Table 10.1). With the possible exception of BeH2 (for which good data are not available) all the s-block hydrides are exergonic (∆fGO< 0) and therefore thermodynamically stable with respect to their elements at room temperature. The trend is erratic in Group 13, in that only AlH3 is exergonic at room temperature. In all the other groups of the p block, the simple hydrogen compounds of the first members of the groups (CH4, NH3, H2O, and HF) are exergonic but the analogous compounds of their congeners become progressively less stable down the group, a trend that is illustrated by decreasing E-H bond energies (Fig. 10.3). The heavier hydrides become more stable on going from Group 14 across to the halogens. For example, SnH4 is highly endergonic (∆fGO> 0) whereas HI is barely so. These thermodynamic trends can be traced to the variation in atomic properties. The HH bond is the strongest single homonuclear bond known (apart from D-D or T-T bonds) and in order for a compound to be exergonic and stable with respect to its elements, it needs to have E H bonds that are even stronger than HH. For molecular hydrides of the p-block elements, bonding is strongest with the Period 2 elements and becomes progressively weaker down each group. The weak bonds formed by the heavier p-block elements are due to the poor overlap between the relatively compact H1s orbital and the more diffuse s and p orbitals of their atoms. Although d-block elements do not form binary molecular compounds, many complexes contain one or more hydride ligands. Metal hydrogen bond strengths in the d block increase down a group because the 3d orbitals are too contracted to overlap well with the H1s orbital and better overlap is afforded by 4d and 5d orbitals.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


