
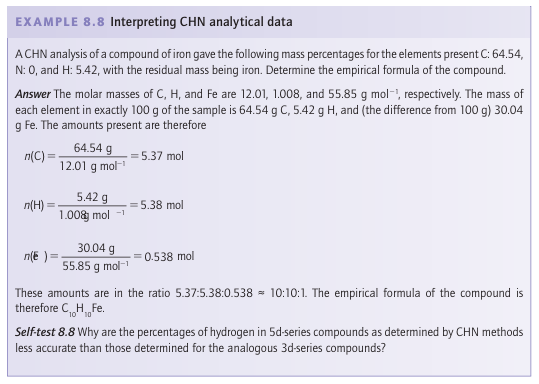
CHN analysis
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص246-247
الجزء والصفحة:
ص246-247
 2025-09-02
2025-09-02
 372
372
CHN analysis
Key point: The carbon, hydrogen, nitrogen, oxygen, and sulfur content of a sample can be determined by high-temperature decomposition. Instruments are available that allow automated analysis of C, H, N, O, and S. Figure 8.40 shows the arrangement for an instrument that analyses for C, H, and N, sometimes referred to as CHN analysis. The sample is heated to 900ºC in oxygen and a mixture of carbon dioxide, carbon monoxide, water, nitrogen, and nitrogen oxides is produced. A stream of helium sweeps the products into a tube furnace at 750ºC, where copper reduces nitrogen oxides to nitrogen and removes oxygen. Copper oxide converts carbon monoxide to carbon dioxide. The resulting mixture is analysed by passing it through a series of three thermal conductivity detectors. The first detector measures hydrogen and then water is removed in a trap. At the second detector the carbon is measured, and carbon dioxide is removed in a second trap. The remaining nitrogen is measured at the third detector. The data obtained from this technique are reported as mass percentage C, H, and N. Oxygen may be analysed if the reaction tube is replaced with a quartz tube filled with carbon that has been coated with catalytic platinum. When the gaseous products are swept through this tube, the oxygen is converted to carbon monoxide, which is then converted to carbon dioxide by passage over hot copper oxide. The rest of the procedure is the same as described above. Sulfur can be measured if the sample is oxidized in a tube filled with cop per oxide. Water is removed by trapping in a cool tube and the sulfur dioxide is determined at what is normally the hydrogen detector.
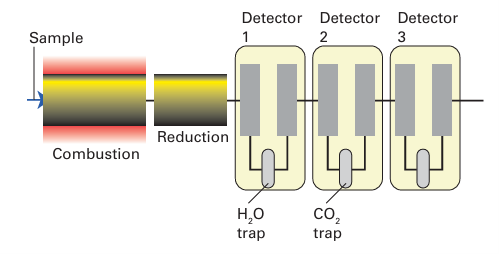
Figure 8.40 The layout of the apparatus used for CHN analysis.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


