
The exclusion rule
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
188
الجزء والصفحة:
188
 2025-08-30
2025-08-30
 414
414
The exclusion rule
The three-atom nonlinear molecule H2O has 3x3 – 6 = 3 vibrational modes (Fig. 6.14). It should be intuitively obvious (and can be confirmed by group theory) that all three vibrational displacements lead to a change in the dipole moment. It follows that all three modes of this C2v molecule are IR active. It is much more difficult to judge intuitively whether or not a mode is Raman active because it is hard to know whether a particular distortion of a molecule results in a change of polarizability (although modes that result in a swelling of the molecule such as the symmetric stretch of CO2 are good prospects). This difficulty is partly overcome by the exclusion rule, which is sometimes helpful: If a molecule has a centre of inversion, none of its modes can be both IR and Raman active. (A mode may be inactive in both.)
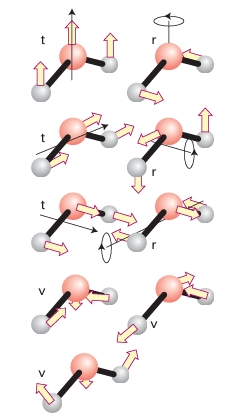
Figure 6.14 An illustration of the counting procedure for displacements of the atoms in a nonlinear molecule.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


