
Basic solvents
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص141-142
الجزء والصفحة:
ص141-142
 2025-08-28
2025-08-28
 392
392
Basic solvents
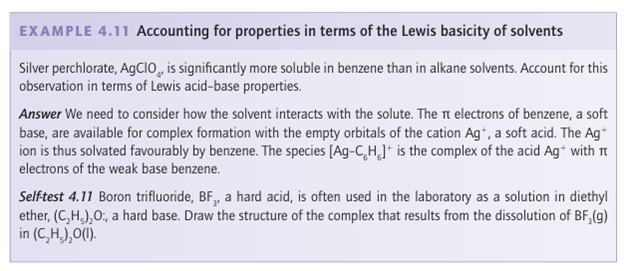
Key points: Basic solvents are common; they may form complexes with the solute and participate in displacement reactions. Solvents with Lewis base character are common. Most of the well-known polar solvents, including water, alcohols, ethers, amines, dimethylsulfoxide (DMSO, (CH3)2SO), dimethylformamide (DMF, (CH3)2NCHO), and acetonitrile (CH3CN), are hard Lewis bases. Dimethylsulfoxide is an interesting example of a solvent that is hard on account of its O donor atom and soft on account of its S donor atom. Reactions of acids and bases in these solvents are generally displacements:

 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


