
Anhydrous sulfuric acid
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص130-131
الجزء والصفحة:
ص130-131
 2025-08-27
2025-08-27
 472
472
Anhydrous sulfuric acid
Key point: The autoionization of anhydrous sulfuric acid is complex, with several competing side reactions. Anhydrous sulfuric acid is an acidic solvent. It has a high relative permittivity and is vis cous because of extensive hydrogen bonding (Section 10.6). Despite this association the solvent is appreciably autoionized at room temperature. The major autoionization is
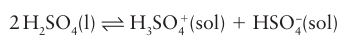
However, there are secondary autoionizations and other equilibria, such as
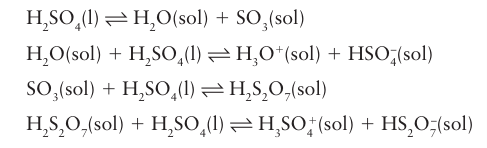
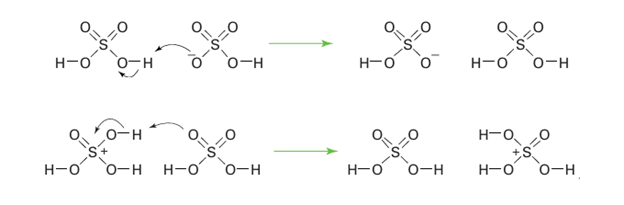
The high viscosity and high level of association through hydrogen bonding would usually lead to low ion mobilities. However, the mobilities of H3SO4 and HSO4– are comparable to those of H3O and OH– in water, indicating that similar proton transfer mechanisms are taking place. The main species taking part are H3SO4 and HSO4–:
Most strong oxo acids accept a proton in anhydrous sulfuric acid and are thus bases:
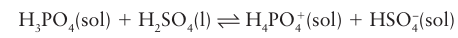
An important reaction is that of nitric acid with sulfuric acid to generate the nitronium ion, NO2, which is the active species in aromatic nitration reactions:
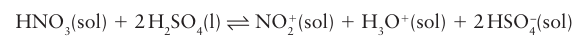
Some acids that are very strong in water act as weak acids in anhydrous sulfuric acids, for example perchloric acid, HClO4, and fluorosulfuric acid, HFSO3.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


