

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Spin-Spin Splitting - What We Observe
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
12-1-2022
3262
Spin-Spin Splitting - What We Observe
If you look at the nmr spectrum of ethanol, CH3CH2OH, in Figure 9-23, you will see that the CH2 resonance is actually a group of four lines and the CH3 resonance is a group of three lines. This three-four line pattern for the grouping CH3CH2X(X≠H) also is evident in the 220MHz spectrum of 2-methyl-2-butanol (Figure 9-27) and in the 60MHz spectrum of ethyl iodide (Figure 9-32).
Why do certain proton resonances appear as groups of equally spaced lines rather than single resonances? The facts are that nonequivalent protons on contiguous carbons 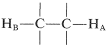 , such as ethyl derivatives CH3CH2X, interact magnetically to "split" each other's resonances. This multiplicity of lines produced by the mutual interaction of magnetic nuclei is called "spin-spin splitting", and while it complicates nmr spectra, it also provides valuable structural information, as we shall see.
, such as ethyl derivatives CH3CH2X, interact magnetically to "split" each other's resonances. This multiplicity of lines produced by the mutual interaction of magnetic nuclei is called "spin-spin splitting", and while it complicates nmr spectra, it also provides valuable structural information, as we shall see.
An example of a complex proton spectrum is that of ethyl iodide (Figure 9-32). To a first approximation, the two main groups of lines appear as equally spaced sets of three and four lines, arising from what are called "first-order spin-spin interactions". Matters are further complicated by additional splitting of the "three-four" pattern of ethyl iodide, as also can be seen in Figure 9-32. This additional splitting is called "second-order" splitting.
When there are so many lines present, how do we know what we are dealing with? From where to we measure the chemical shift in a complex group of lines?
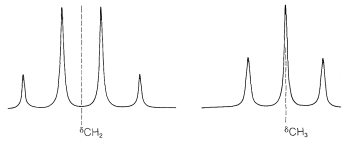
Figure 9-32: High-resolution nmr spectrum of ethyl iodide, CH3CH2I, at 60MHz relative to TMS, 0.00ppm. The first-order splitting pattern is seen in the well-separated "three-four" line pattern for the CH3−CH2 resonances. The second-order splitting is the additional fine structure superimposed on the three-four pattern.
First, the chemical shift normally is at the center of the group of lines corresponding to first-order splitting. In ethyl iodide, the chemical shift of the methyl protons is in the center of the quartet:
Second, the chemical shift can be recognized by the fact that it is directly proportional to the transmitter frequency, ν. If we double ν, the chemical shifts double. In contrast, the first-order spin-spin splittings remain the same. By this we mean that the magnitude (in Hz) of the spacing between the lines of a split resonance is independent of the transmitter frequency, νν. This spacing corresponds to what is called the spin-spin coupling constant, or simply the coupling constant, and is symbolized by J.
Figure 9-27).
Third, the second-order splitting tends to disappear with increasing transmitter frequency. For ethyl iodide (Figure 9-32), the second-order splitting at 60MHz is barely discernible at 100MHz and disappears at 200MHz. This also can be seen to occur for the three-four splitting pattern of 2-methyl-2-butanol as a function of ν (Figure 9-27).
The next question is how can we understand and predict what spin-spin splitting patterns will be observed? And how do they give us structural information? The important point is that the multiplicity of lines for protons of a given chemical shift often is seen to be (n+1), in which nn is the number of protons on the contiguous carbons. For example, the CH2 resonance of the ethyl group of ethyl iodide is a quartet of lines because of the spin-spin interaction with the neighboring three protons (n=3) of the methyl group. Likewise, the CH3 group is a triplet of lines because of spin-spin interactions with the two protons (n=2) of the methylene group.
Figure 9-33, where X and Y are groups that give no spin interactions with the protons. The value of these patterns, when observed, lies in the way that they indicate the number of equivalent protons on contiguous carbons. For instance, a two-three line pattern, where the two-part has an integrated intensity twice that of the three-part, suggests the grouping XCH2−CHY2.
Figure 9-33: Schematic proton nmr spectra, X and Y are nonmagnetic nuclei. For 2-propane derivatives, as at the top, the (CH3) resonances are double because of the splitting produced by the single proton on C2. For the ethane derivatives, the right set of lines is always a triplet when observable because of the two protons of the X−CH2− group. We assume here that the chemical shifts of the CHnY3−n protons are independent of the number of Y substituents.
The ratios of the line intensities in the spin-spin splitting patterns of Figure 9-33 usually follow simple rules. A doublet appears as two lines of equal intensity; a triplet as three lines in the ratio 1:2:1; a quartet as four lines in the ratio 1:3:3:1; a quintet as 1:4:6:4:1, and so on. The intensities follow the binomial coefficients for (x+y)n(x+y)n, where nn is the number of protons in the splitting group. Thus when n=4n=4, we have x4+4x3y+6x2y2+4xy3+y4, or 1:4:6:4:1.
The spectrum of (CH3O)2CHCH3 (Figure 9-34) provides an excellent example of how nmr shows the presence of contiguous protons. The symmetrical doublet and 1:3:3:1 quartet are typical of the interaction between a single proton and an adjacent group of three, that is, 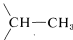 . The methyl protons of the (CH3O) groups are too far from the others to give demonstrable spin-spin splitting; thus they appear as a single six-proton resonance.
. The methyl protons of the (CH3O) groups are too far from the others to give demonstrable spin-spin splitting; thus they appear as a single six-proton resonance.
Figure 9-34: Proton nmr spectrum of 1,1-dimethoxyethane (dimethyl acetal), (CH3O)2CHCH3, at 60MHz relative to TMS, 0.00ppm.
In general, the magnitude of the spin-spin splitting effect of one proton on another proton (or group of equivalent protons) depends on the number and kind of intervening chemical bonds and on the spatial relationships between the groups. For simple systems without double bonds and with normal bond angles, we usually find for nonequivalent protons (i.e., having different chemical shifts):
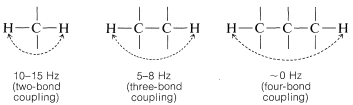
Where restricted rotation or double- and triple-bonded groups are involved, widely divergent splittings are observed. For double bonds, the two-bond couplings between two nonequivalent hydrogens located on one end are characteristically small, while the three-bond couplings in −HC=CH− are larger, especially for the trans configuration:
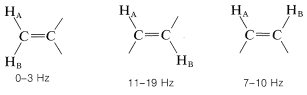
Coupling through four or more bonds is significant for compounds with double or triple bonds. Examples of these so-called long-range couplings and some other useful splitting values follow:
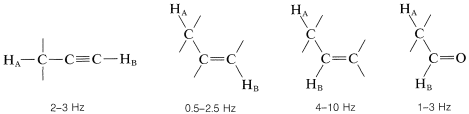
Finally, chemically equivalent protons do not split each other's resonances.
 الاكثر قراءة في التشخيص العضوي
الاكثر قراءة في التشخيص العضوي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















