

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
More on Hybrid Bond Orbitals and Molecular Geometry
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
31-12-2021
2822
More on Hybrid Bond Orbitals and Molecular Geometry
A summary of the directional character of the ss-pp hybrid atomic orbitals discussed so far is given in Table 6-2. By referring to this table, it usually is possible to deduce the nature of the bonding orbitals for most organic compounds from the molecular geometry, if this is known, Thus a tetrahedral molecule AX4 with four attached ligands uses sp3 hybrid orbitals localized on atom A; a planar triangular molecule AX3 with three attached ligands at angles of 120o is sp2 hybridized at atom A; a linear molecule AX2 with two ligands is sp hybridized at A.
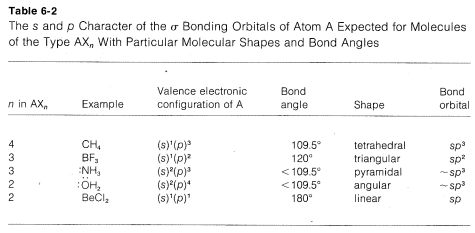
Applying the converse of these rules, one should be able to predict molecular geometry by making reasonable assumptions as to the state of hybridization for each atom in the molecule. Obviously in doing this we have to take account of unshared electron pairs. Prediction is easy if unshared pairs are absent. Thus, four attached ligands, as in CH4, CCl4, or BF4⊖, imply sp3 hybridization at the central atom and therefore a tetrahedral arrangement of ligands. Three ligands, as bonded to carbon in CH3⊕ or to boron in BF3, imply sp2 hybridization for the central atom and a planar triangular arrangement if ligands. Two ligands, as in CO2, imply spsp hybridization and linear geometry.
In many of our later discussions of organic reactions, we will be concerned with cationic, radical, and anionic carbon species that are substitution products of CH3⊕, CH3⋅, and CH3:⊖. Because of the importance of these entities, you should know how to formulate them and related substances, such as ⊖:N:H2, with atomic orbitals. Perhaps the most straightforward way is to start from CH4CH4 and see what changes in the C−H bonds we would expect as the result of the hypothetical processes: CH4→CH3:⊖+H⊕, CH4→CH3⊕+H:⊖, and CH4→CH3⋅+H⋅CH4→CH3⋅+H⋅.
Methane is tetrahedral with sp3 carbon bonding orbitals. Removal of H⊕ gives CH3:⊖, which corresponds in electronic structure to H3N: and, for the same reasons, should have a pyramidal shape with nearly tetrahedral H−C−H angles. Removal of H:⊖ from CH4 to give CH3⊕ with six bonding electrons, suggests a change to sp2 bonding orbitals for the carbon and planar geometry with H−C−H angles of 120o.
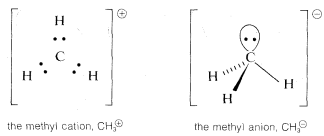
The radical, CH3⋅ presents a special problem. We can think of it as being formed by the loss of H⋅H⋅ from CH4CH4, by adding an electron to planar CH3⊕, or by removing an electron from pyramidal CH3:⊖. We can formulate CH3⋅ with Sp2 orbitals for the C−H bonds and the extra electron in a p orbital, or with sp3 orbitals for the C−H bonds and the extra electron in an sp3 orbital:

The actual structure of CH3⋅ has the hydrogens and carbons in a plane (left). Therefore it appears that the repulsions between the bonding electron pairs is greater than the repulsions between the extra electron and the bonding pairs. The actual structure corresponds to the one in which the bonding pairs are as far apart as possible.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















