

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Combustion. Heats of Reaction. Bond Energies
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
23-12-2021
2433
Combustion. Heats of Reaction. Bond Energies
All hydrocarbons are attacked by oxygen at elevated temperatures and, if oxygen is in excess, complete combustion occurs to carbon dioxide and water:
[ CH_4 + 2O_2 ightarrow CO_Edit2 + 2H_2O]
The heat evolved in this process - the heat of the combustion reaction, ΔH - is a measure of the amount of energy stored in the C−C and C−H bonds of the hydrocarbon compared to the energy stored in the products, carbon dioxide and water. It can be measured experimentally with considerable accuracy and generally is reported as ΔH0ΔH0 the amount of heat (in kilocalories) liberated on complete combustion of one mole of hydrocarbon when the reactants and the products are in standard states, and at the same temperature, usually 25o. Not all chemical reactions that occur spontaneously liberate heat - some actually absorb heat. By convention, ΔH0 is given a negative sign when heat is evolved (exothermic reaction) and a positive sign when heat is absorbed (endothermic reaction). The heat evolved or absorbed also is called the enthalpy change.
For the combustion of 1mol1mol of methane at 15o, we find by experiment (corrected from constant volume to constant pressure, if necessary) that the reaction is exothermic by 212.8 kcal. This statement can be expressed as follows:
with ΔHo=−212.8kcal
The symbol (g) denotes that the reactants and products are in the gaseous state except for the water, which is liquid (l). If we wish to have ΔH0 with gaseous water H2O(g) as the product we have to make a correction for the heat of vaporization of water (10.5kcalmol−1at 25o):
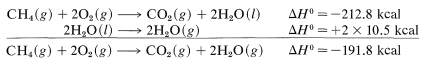
The task of measuring the heats of all chemical reactions is a formidable one and about as practical as counting grains of sand on the beach. However, it is of practical interest to be able to estimate heats of reaction, and this can be done quite simply with the aid of bond energies. The necessary bond energies are given in Table 4-3, and it is important to notice that they apply only to complete dissociation of gaseous substances to gaseous atoms at 25oC. Also, they do not apply, without suitable corrections, to many compounds, such as benzene, that have more than one double bond.
To calculate ΔH0 for the combustion of one mole of methane, first we break bonds as follows, using 98.7kcalmol−1 for the energy of each of the
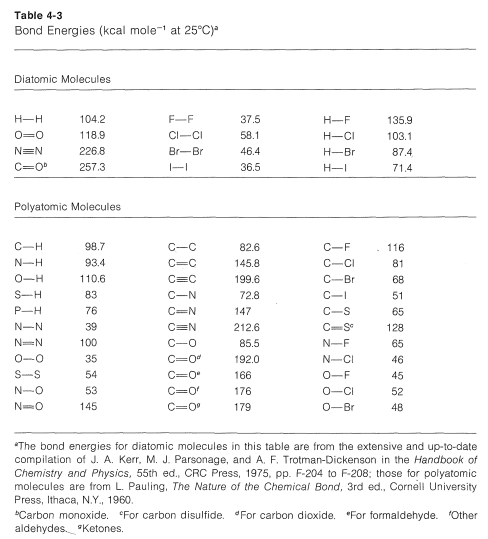
C−HC−H bonds,
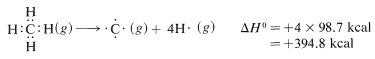
and then 118.9kcalmol−1118.9kcalmol−1 for the energy of the double bond in oxygen:
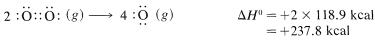
Then we make bonds, using 192kcalmol−1192kcalmol−1 for each O=CO=C bond in carbon dioxide,

and 110.6kcalmol−1110.6kcalmol−1 for each of the H−O bonds in water:
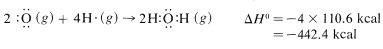
The net sum of these ΔH0 values is 394.8+237.8−384.0−442.4=−193.8kcal, which is reasonably close to the value of −191.8kcal for the heat of combustion of one mole of methane determined experimentally.
The same type of procedure can be used to estimate ΔH0 values for many other kinds of reactions of organic compounds in the vapor phase at 25o. Moreover, if appropriate heats of vaporization are available, it is straightforward to compute ΔH0 for vapor-phase reactions of substances which are normally liquids or solids at 25o.
It is important to recognize that the bond energies listed in Table 4-3 for all molecules other than diatomic molecules are average values. That the C−H bond energy is stated to be 98.7kcal does not mean that, if the hydrogens of methane were detached one by one, 98.7kcal would have to be put in at each step. Actually, the experimental evidence is in accord with quite different energies for the separate dissociation steps:
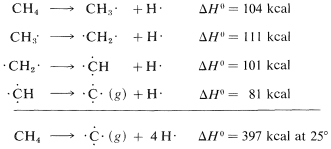
The moral is that we should try to avoid using the bond energies in Table 4-3 as a measure of ΔH0ΔH0 for the dissociation of just one bond in a polyatomic molecule. For this we need what are called bond-dissociation energies, some of which are given in Table 4-6. The values given have been selected to emphasize how structure influences bond energy. Thus, C−H bond energies in alkanes decrease in the order primary >> secondary >> tertiary; likewise, C−H bonds decrease in strength along the series C≡C−H>C=C−H>C−C−HC≡C−H>C=C−H>C−C−H.
How accurate are ΔH0 values calculated from bond energies? Generally quite good provided nonbonded interactions between the atoms are small and the bond angles and distances are close to the normal values . A few examples of calculated and experimental heats of combustion of some hydrocarbons are given in Table 4-4. Negative discrepancies represent heats of combustion smaller than expected from the average bond energies and positive values correspond to larger than expected heats of combustion.
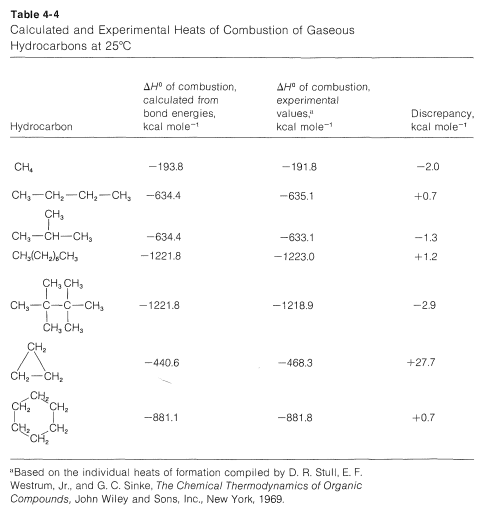
Comparing isomers in Table 4-4, we see that 2-methylpropane and 2,2,3,3-tetramethylbutane give off less heat when burned than do butane and octane, and this is a rather general characteristic result of chain branching.
Cyclopropane has a ΔH0 of combustion of 27.7kcalmol−1 greater than expected from bond energies, and this clearly is associated with the abnormal C−C−C bond angles in the ring.
For cyclohexane, which has normal bond angles, the heat of combustion is close to the calculated value.
In this book we use kilocalories in place of the presently recommended (SI) joules for units of energy. As of the date of writing, it is not clear just how general the use of the joule will become among chemists. To convert calories to joules (or kcal to kJ), multiply by 4.184.
33You may wonder how a reaction, such as combustion of methane, can occur at 25o. The fact is that the reaction can be carried out at any desired temperature. The important thing is that the ΔH0 value we are talking about here is the heat liberated or absorbed when you start with the reactants at 25o and finish with the products at 25o. As long as ΔH0 is defined this way, it does not matter at what temperature the reaction actually occurs. Standard states for gases are 1atm1atm partial pressure. Standard states for liquids or solids usually are the pure liquid or solid at 1atm1atm external pressure.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















