

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Structure and Nomenclature of Aromatic Compounds
المؤلف:
LibreTexts Project
المصدر:
................
الجزء والصفحة:
.................
11-9-2020
1991
Structure and Nomenclature of Aromatic Compounds
Historically, benzene-like substances were called aromatic hydrocarbons because they had distinctive aromas. Today, an aromatic compound is any compound that contains a benzene ring or has certain benzene-like properties (but not necessarily a strong aroma). You can recognize the aromatic compounds in this text by the presence of one or more benzene rings in their structure. Some representative aromatic compounds and their uses are listed in Table 1 , where the benzene ring is represented as C6H5.
| Name | Structure | Typical Uses |
|---|---|---|
| aniline | C6H5–NH2 | starting material for the synthesis of dyes, drugs, resins, varnishes, perfumes; solvent; vulcanizing rubber |
| benzoic acid | C6H5–COOH | food preservative; starting material for the synthesis of dyes and other organic compounds; curing of tobacco |
| bromobenzene | C6H5–Br | starting material for the synthesis of many other aromatic compounds; solvent; motor oil additive |
| nitrobenzene | C6H5–NO2 | starting material for the synthesis of aniline; solvent for cellulose nitrate; in soaps and shoe polish |
| phenol | C6H5–OH | disinfectant; starting material for the synthesis of resins, drugs, and other organic compounds |
| toluene | C6H5–CH3 | solvent; gasoline octane booster; starting material for the synthesis of benzoic acid, benzaldehyde, and many other organic compounds |
Example 1
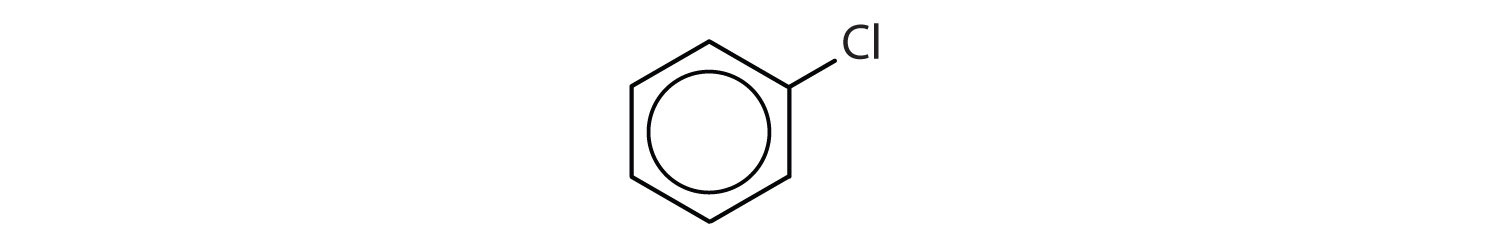
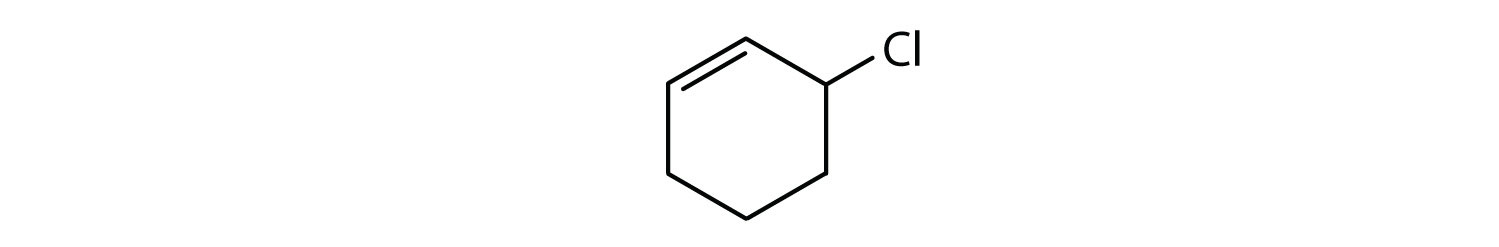
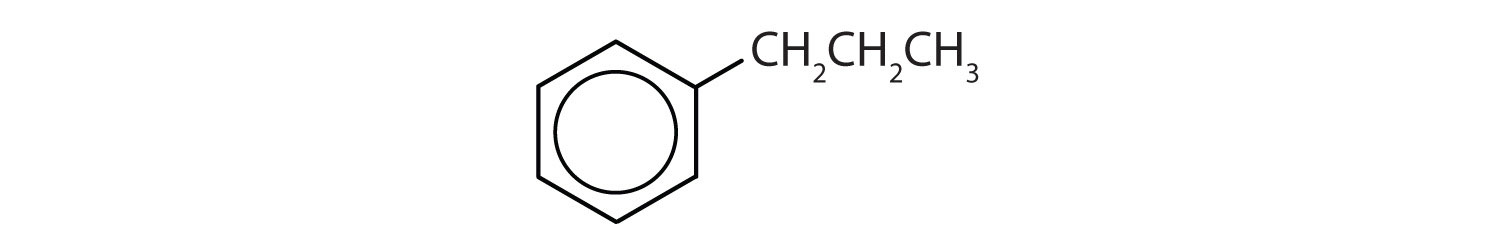
Which compounds are aromatic?
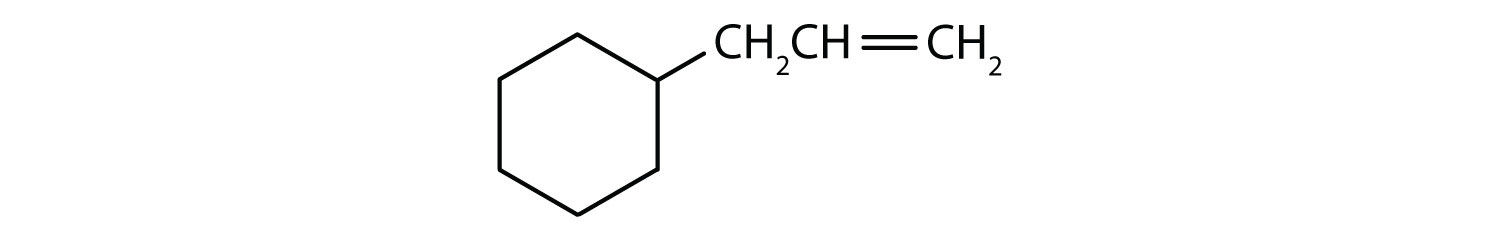
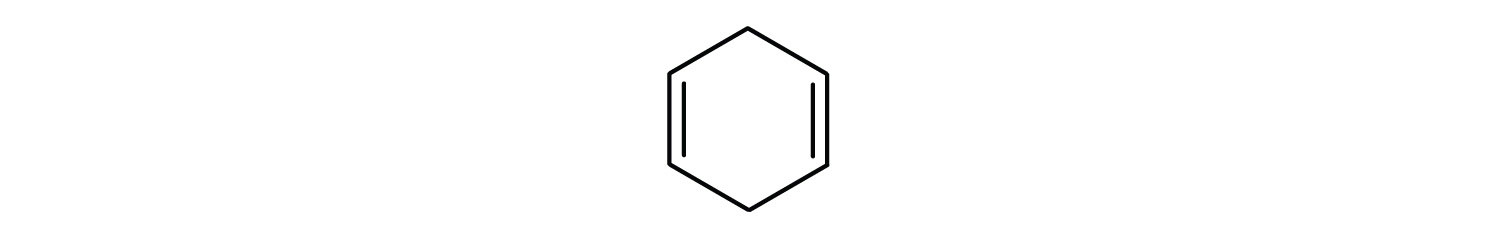
Solution
- The compound has a benzene ring (with a chlorine atom substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
- The compound has a benzene ring (with a propyl group substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
Exercise 1
Which compounds are aromatic?
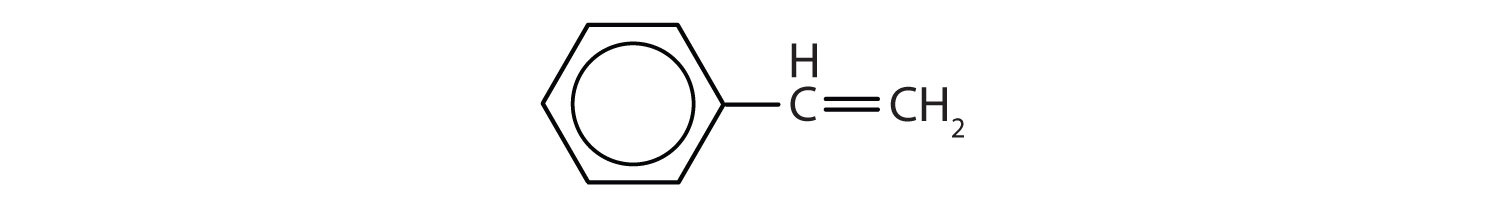
In the International Union of Pure and Applied Chemistry (IUPAC) system, aromatic hydrocarbons are named as derivatives of benzene. Figure 1
shows four examples. In these structures, it is immaterial whether the single substituent is written at the top, side, or bottom of the ring: a hexagon is symmetrical, and therefore all positions are equivalent.
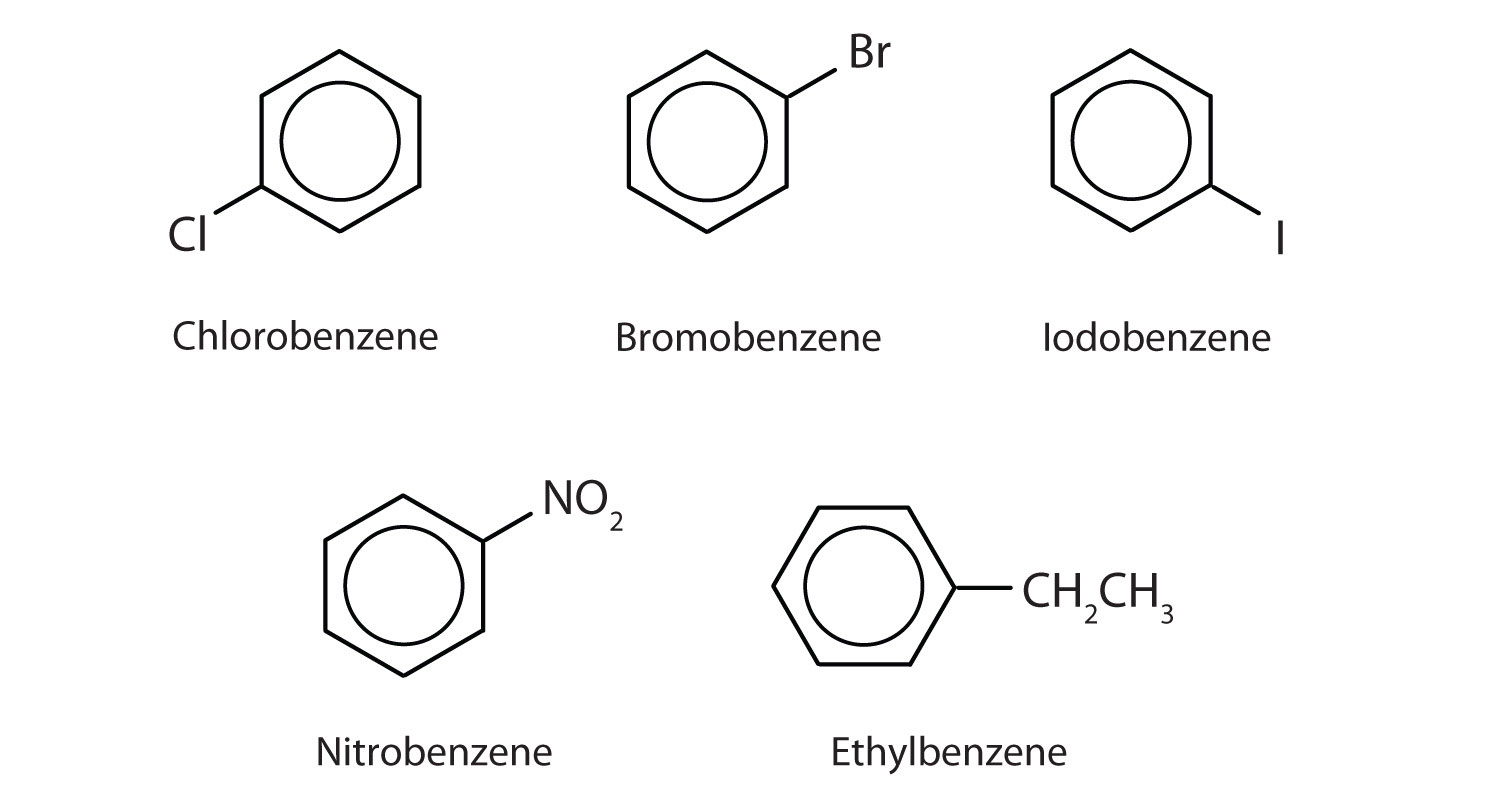
Figure 1 : Some Benzene Derivatives. These compounds are named in the usual way with the group that replaces a hydrogen atom named as a substituent group: Cl as chloro, Br as bromo, I as iodo, NO2 as nitro, and CH3CH2 as ethyl.
Although some compounds are referred to exclusively by IUPAC names, some are more frequently denoted by common names, as is indicated in Table 1.
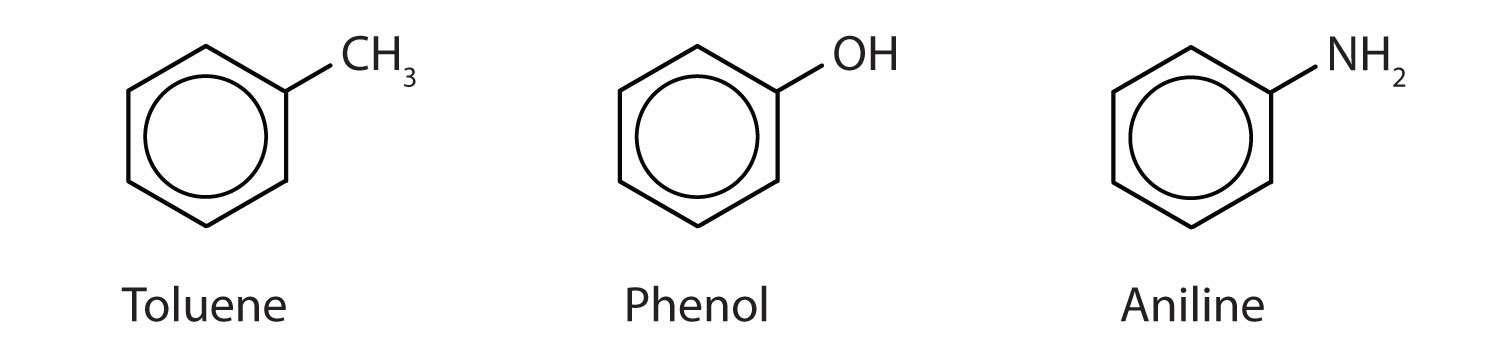
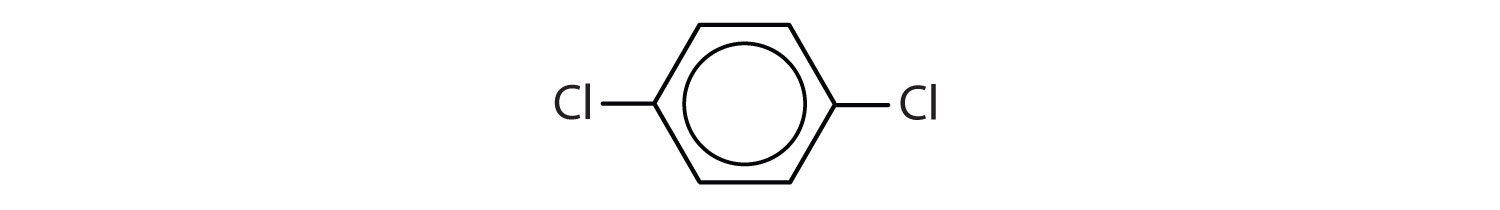
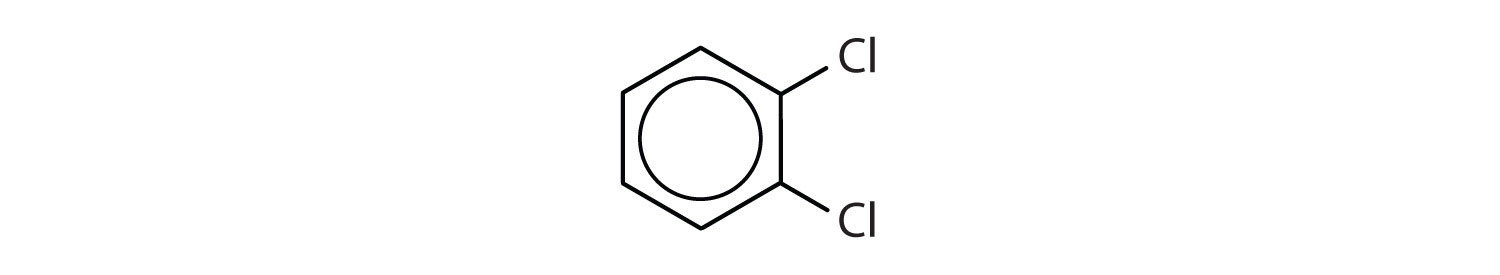
When there is more than one substituent, the corners of the hexagon are no longer equivalent, so we must designate the relative positions. There are three possible disubstituted benzenes, and we can use numbers to distinguish them (Figure 2). We start numbering at the carbon atom to which one of the groups is attached and count toward the carbon atom that bears the other substituent group by the shortest path.
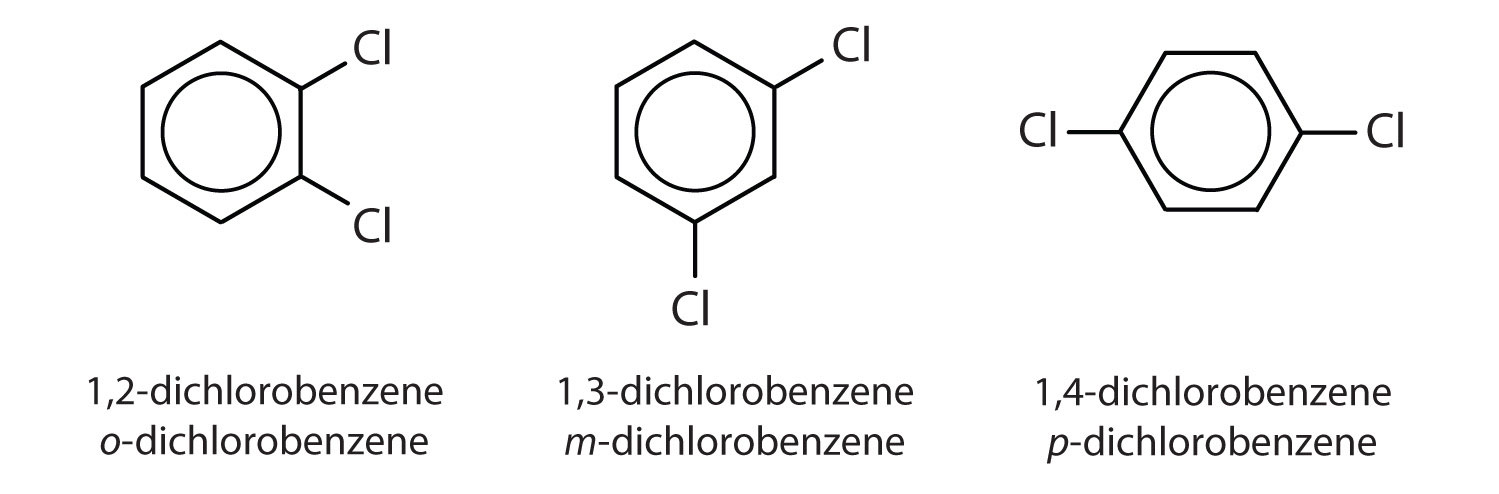
Figure 2 : The Three Isomeric Dichlorobenzenes
In Figure 2 , common names are also used: the prefix ortho (o-) for 1,2-disubstitution, meta (m-) for 1,3-disubstitution, and para (p-) for 1,4-disubstitution. The substituent names are listed in alphabetical order. The first substituent is given the lowest number. When a common name is used, the carbon atom that bears the group responsible for the name is given the number 1:
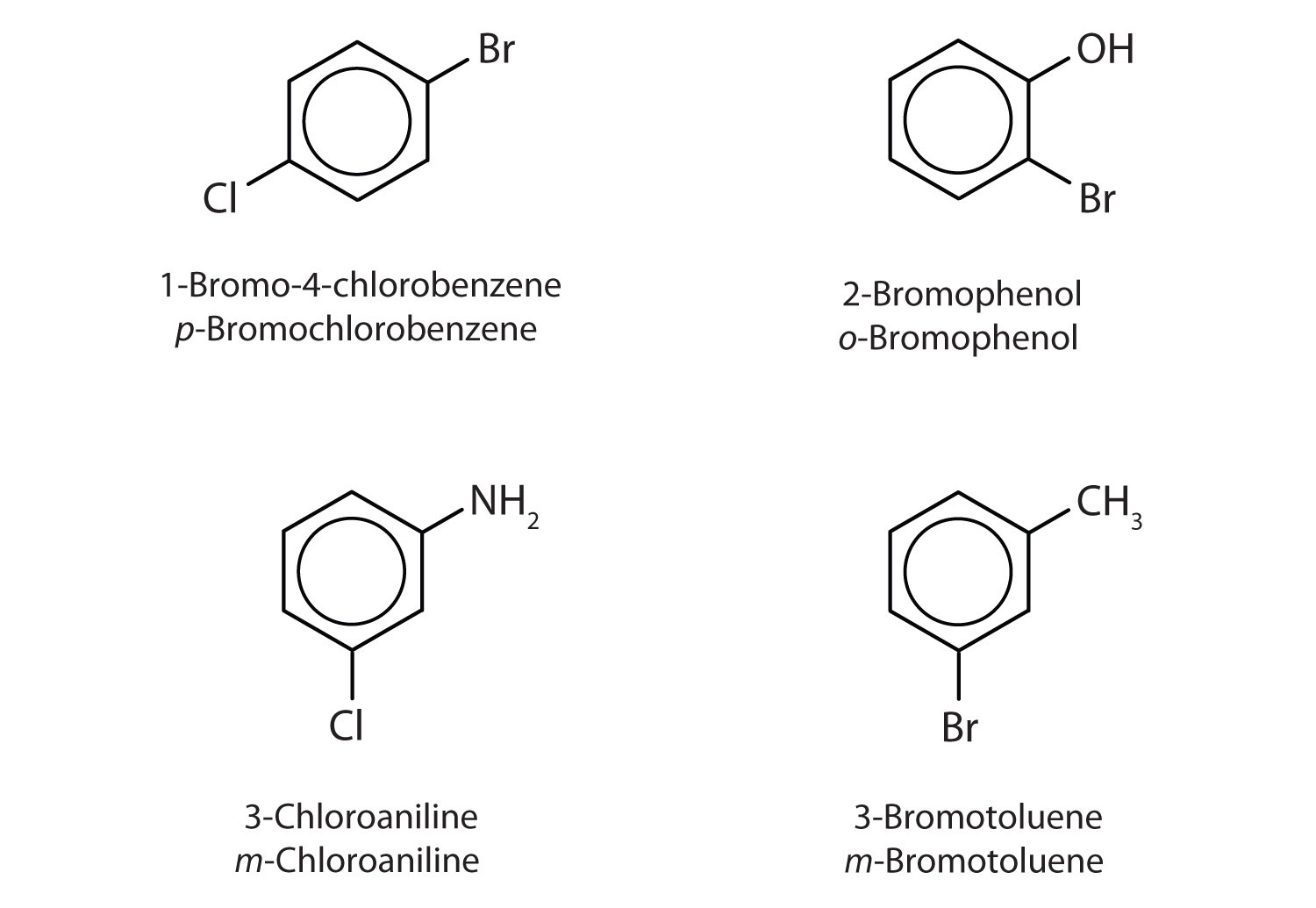
Example 2
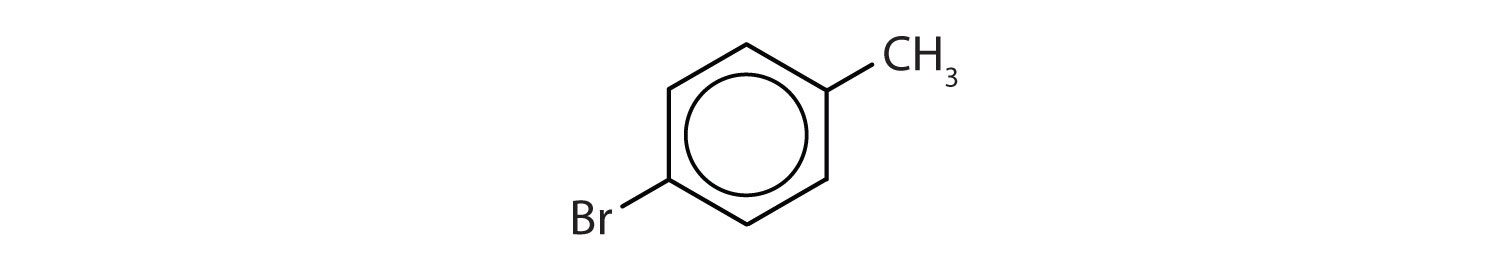
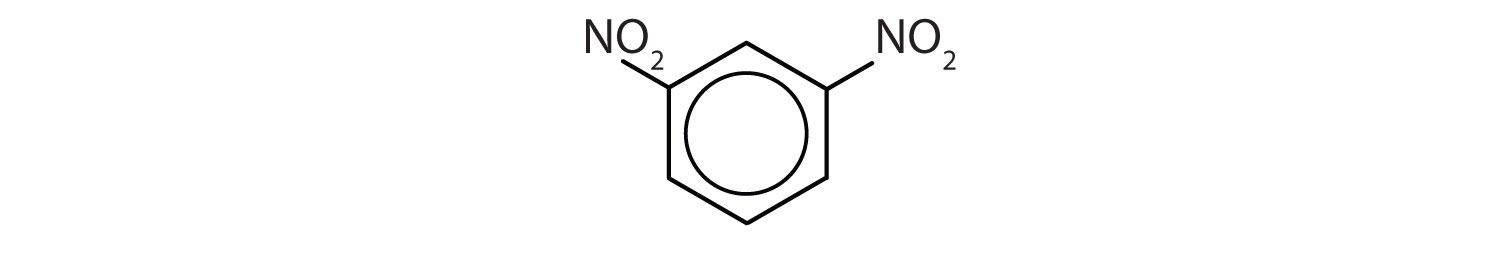
Name each compound using both the common name and the IUPAC name.
Solution
- The benzene ring has two chlorine atoms (dichloro) in the first and second positions. The compound is o-dichlorobenzene or 1,2-dichlorobenzene.
- The benzene ring has a methyl (CH3) group. The compound is therefore named as a derivative of toluene. The bromine atom is on the fourth carbon atom, counting from the methyl group. The compound is p-bromotoluene or 4-bromotoluene.
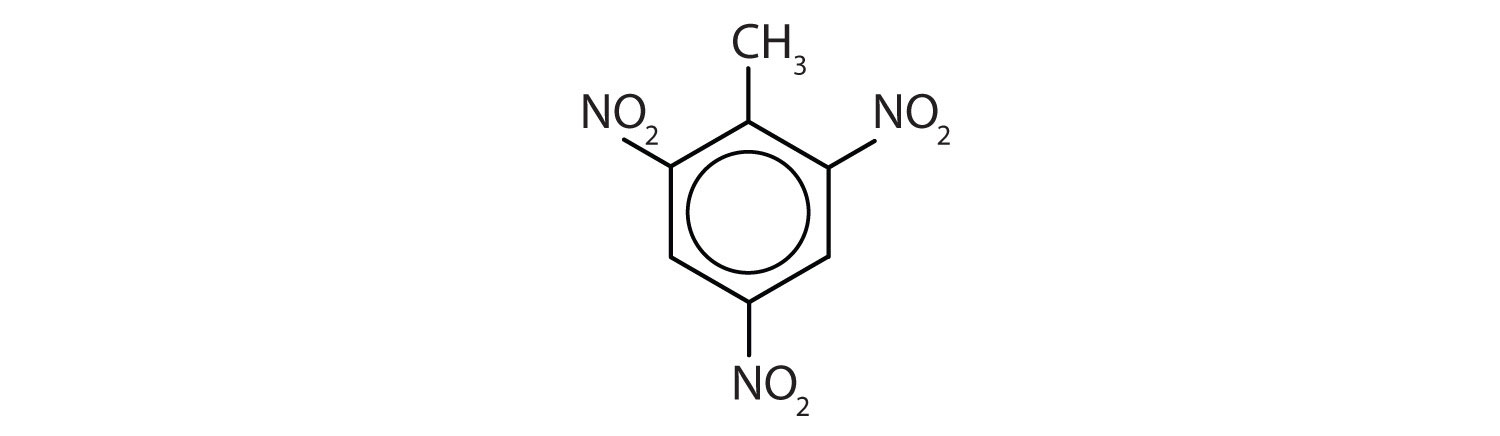
- The benzene ring has two nitro (NO2) groups in the first and third positions. It is m-dinitrobenzene or 1,3-dinitrobenzene.
Note: The nitro (NO2) group is a common substituent in aromatic compounds. Many nitro compounds are explosive, most notably 2,4,6-trinitrotoluene (TNT).
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















