


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-11-2018
Date: 10-12-2018
Date: 9-10-2018
|
Oxides of selenium and tellurium
Selenium and tellurium dioxides are white solids obtained by direct combination of the elements. The polymorph of TeO2 so formed is α-TeO2, whereas β-TeO2 occurs naturally as the mineral tellurite. Both forms of TeO2 contain structural units 1.1 which are connected by shared O atoms into a three-dimensional lattice in α-TeO2, and into a sheet structure in the β-form. The structure of SeO2 consists of chains (1.2) in which the Se centres are in trigonal pyramidal environments. Whereas SeO2 sublimes at 588 K, TeO2 is an involatile solid (mp 1006 K). In the gas phase, SeO2 is monomeric with structure 1.3. The trends in structures of the dioxides of S, Se and Te and their associated properties (e.g. mp, volatility) reflect the increase in metallic character on descending group 16.

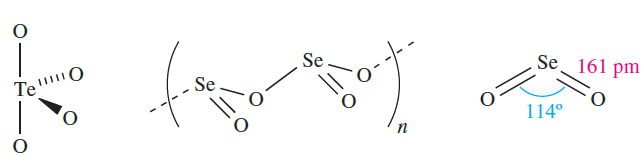
(1.1) (1.2) (1.3)
Selenium dioxide is very toxic and is readily soluble in water to give selenous acid, H2SeO3. It is readily reduced, e.g. by hydrazine, and is used as an oxidizing agent in organic reactions. The α-form of TeO2 is sparingly soluble in water, giving H2TeO3, but is soluble in aqueous HCl and alkali. Like SeO2, TeO2 is a good oxidizing agent. Like SO2, SeO2 and TeO2 react with KF (see equation 15.85). In solid K[SeO2F], weak fluoride bridges link the [SeO2F]- ions into chains. In contrast, the tellurium analogue contains trimeric anions (structure 1.4, see worked example below). Selenium trioxide is a white, hygroscopic solid. It is difficult to prepare, being thermodynamically unstable with respect to SeO2 and O2 (ΔfHo(298 K): SeO2 = _225; SeO3 = _184 kJ mol-1). It may be made by reaction of SO3 with K2SeO4 (a salt of selenic acid). Selenium trioxide decomposes at 438 K, is soluble in water, and is a stronger oxidizing agent than SO3. In the solid state, tetramers (1.5) are present.

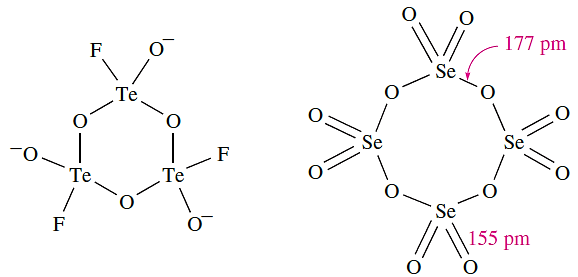
(1.4) (1.5)
Tellurium trioxide (the a-form) is formed by dehydrating telluric acid (equation 1.1). It is an orange solid which is insoluble in water but dissolves in aqueous alkali, and is a very powerful oxidizing agent. On heating above 670 K, TeO3 decomposes to TeO2 and O2. The solid state structure of TeO3 is a three-dimensional lattice in which each Te(VI) centre is octahedrally sited and connected by bridging O atoms.

 (1.1)
(1.1)
Worked example
Selenium and tellurium oxides and their derivatives Diagram 1.4 shows a representation of the structure of [Te3O6F3]3- . The coordination environment of the Te atom is not tetrahedral. Rationalize this observation.
Apply VSEPR theory to structure 1.4: Te is in group 16 and has six valence electrons. The formation of Te_F and three Te_O bonds (terminal and two bridging O atoms) adds four more electrons to the valence shell of Te. In [Te3O6F3]3- , each Te centre is surrounded by five electron pairs, of which one is a lone pair. Within VSEPR theory, a trigonal bipyramidal coordination environment is expected.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بالصور: ممثل المرجعية العليا والامين العام للعتبة الحسينية يستقبلون المهنئين القاصدين مرقد الامام الحسين (ع) في عيد الفطر المبارك
|
|
|