
Why Bother?
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 3
الجزء والصفحة:
part 2 , p 3
 10-9-2016
10-9-2016
 2013
2013
Why Bother?
A physicist and an engineer find themselves in a mountain lodge where the only heat is provided by a large woodstove. The physicist argues that
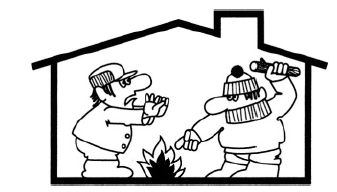
Figure 1.1
they cannot increase the total energy of the molecules in the cabin, and therefore it makes no sense to continue putting logs into the stove. The engineer strongly disagrees (see Figure 1.1), referring to the laws of thermodynamics and common sense. Who is right? Why do we heat the room?
SOLUTION
The physicist is right in saying that the total energy of the molecules in the room cannot be changed. Indeed, the total energy of an ideal gas is

where N is the number of molecules, cv is the heat capacity at constant volume per particle, and τ is the absolute temperature in energy units. In these units,

Since the pressure P in the room stays the same (as does the volume V) and equal to the outside air pressure, we have
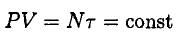
So, the total energy of the gas does not change. However, the average energy of each molecule does, of course, increase, and that is what defines the temperature (and part of the comfort level of the occupants). At the same time, the total number of molecules in the room decreases. In essence, we burn wood to force some of the molecules to shiver outside the room (this problem was first discussed in Nature 141, 908 (1938)).
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


