

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Creating and Maintaining Order Requires Work and Energy
المؤلف:
David L. Nelson, Michael M. Cox
المصدر:
Lehninger Principles of Biochemistry 6th ed 2012
الجزء والصفحة:
6th ed -p23
26-7-2016
2751
Creating and Maintaining Order Requires Work and Energy
DNA, RNA, and proteins are informational macromolecules. In addition to using chemical energy to form the covalent bonds between the subunits in these polymers, the cell must invest energy to order the subunits in their correct sequence. It is extremely improbable that amino acids in a mixture would spontaneously condense into a single type of protein, with a unique sequence. This would represent increased order in a population of molecules; but according to the second law of thermodynamics, the tendency in nature is toward ever-greater disorder in the universe: the total entropy of the universe is continually increasing. To bring about the synthesis of macromolecules from their monomeric units, free energy must be supplied to the system (in this case, the cell). The randomness or disorder of the components of a chemical system is expressed as entropy, S.
Any change in randomness of the system is expressed as entropy change, ΔS, which by convention has a positive value when randomness increases. J. Willard Gibbs, who developed the theory of energy changes during chemical reactions, showed that the freeenergy content, G, of any closed system can be defined in terms of three quantities:

J. Willard Gibbs,
1839–1903
enthalpy, H, reflecting the number and kinds of bonds; entropy, S; and the absolute temperature, T (in degrees Kelvin). The definition of free energy is G = H - TS. When a chemical reaction occurs at constant temperature, the free-energy change, ΔG, is determined by the enthalpy change, ΔH, reflecting the kinds and numbers of chemical bonds and noncovalent interactions broken and formed, and the entropy change, ΔS, describing the change in the system’s randomness:
ΔG = ΔH _ T ΔS
A process tends to occur spontaneously only if ΔG is negative. Yet cell function depends largely on molecules, such as proteins and nucleic acids, for which the free energy of formation is positive: the molecules are less stable and more highly ordered than a mixture of their monomeric components. To carry out these thermodynamically unfavorable, energy-requiring (endergonic) reactions, cells couple them to other reactions that liberate free energy (exergonic reactions), so that the overall process is exergonic: the sum of the freeenergy changes is negative. The usual source of free energy in coupled biological reactions is the energy released by hydrolysis of phosphoanhydride bonds such as those in adenosine triphosphate (ATP; Fig. 1–25). Here, each ℗ represents a phosphoryl group:
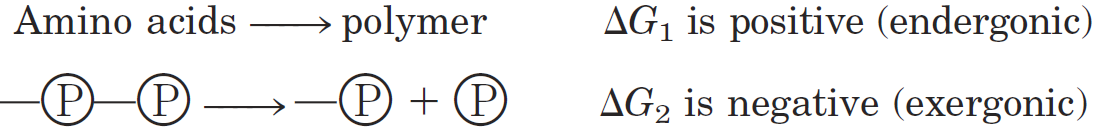
When these reactions are coupled, the sum of ΔG1 and ΔG2 is negative—the overall process is exergonic. By this coupling strategy, cells are able to synthesize and maintain the information-rich polymers essential to life.
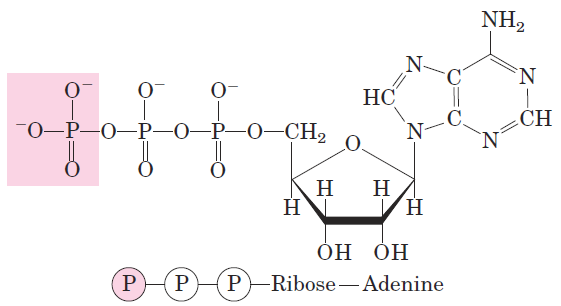
FIGURE 1–25 Adenosine triphosphate (ATP). The removal of the terminal phosphoryl group (shaded pink) of ATP, by breakage of a phosphoanhydride bond, is highly exergonic, and this reaction is coupled to many endergonic reactions in the cell .
 الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
الاكثر قراءة في مواضيع عامة في الكيمياء الحياتية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















