
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Magnetism in Solids
المؤلف:
Roger J Blin-Stoyle, FRS
المصدر:
Physics of Particles, Matter and the Universe
الجزء والصفحة:
p 97
24-5-2016
1505
Magnetism in Solids
We saw that a magnetic field is created when an electric current-a flow of electric charge in the form of electrons passes through a wire. It therefore follows that any electron in an atom possessing some angular momentum and which can be pictured as orbiting about the atomic nucleus, will create a small magnetic field due to its rotation. In addition, electrons, having an intrinsic spin, also create a magnetic field and behave as miniscule magnets. Because of these two sources of a magnetic field in an atom it is to be expected that atoms themselves behave as small magnets. In general this is true except for those atoms in which there are no electrons outside filled shells. This is because in such atoms, for every electron in a filled shell rotating or spinning in one direction, there is another rotating or spinning in the opposite direction. The corresponding magnetic fields then exactly cancel each other and there is no resultant field. This means that in all atoms their associated magnetic fields derive only from those loose electrons outside filled shells; there is no contribution from the filled shells themselves. Solids whose atoms behave as magnets will have magnetic properties and these manifest themselves in two forms-paramagnetism and ferromagnetism.
Paramagnetism. In general, in a solid whose atoms behave as magnets, the magnets are pointing randomly in all directions and there is no resultant magnetic field. However, if an external magnetic field is applied then, just as a magnetic compass needle lines up with the earth’s magnetic field, so it would be expected that the magnetic atoms in the solid will line up and all point in the direction of this field. This does happen to some extent and the result is that the solid becomes magnetized in such a way that the magnetic field due to the solid enhances the applied magnetic field. However the ‘lining up’ is not perfect. A compass needle is generally not subject to any disturbance. On the other hand, the atoms in a solid are being continually disturbed by thermal vibrations. These, of course, become more violent the higher the temperature and it is therefore to be expected that the ‘lining up’ effect will reduce as the temperature is increased. Experimental observation confirms this expectation for non-metals. For metals the situation is somewhat different and more complicated because of the presence of conduction electrons. This enhancement of an applied magnetic field, which occurs in many substances, is known as paramagnetism.
Ferromagnetism. In five elements, of which iron is the best known, it is found that the atomic magnetization results mainly from the spins of electrons in unfilled shells. In the atoms of these elements the most stable configuration-the configuration with lowest energy-is one in which the spins of these electrons are mostly parallel to each other. This arises because with parallel spins the exclusion principle requires each of the electrons to be in a different quantum state. The motion of each electron is therefore different so that they tend to keep well apart from each other. This, in turn, means that they have lower energy in this sort of configuration since, being mainly well separated, they experience less repulsion due to their electric charges. Naturally, the atom generally stays in this lowest energy state. This effect continues to operate when the atoms are assembled in a piece of metal and, indeed, operates between nearby atoms and within the band structure. As a result the spins of the different electrons ‘line up’ without the imposition of an external magnetic field. This means that the magnetic fields of the ‘lined-up’ electrons all point in the same direction, leading to a strong resultant field. Materials which behave in this way are said to be ferromagnetic. Again, as with paramagnetism, this lining up cannot be sustained at high temperatures because of thermal agitation and above a certain temperature-known as the Curie temperature (1043 K for iron) it does not occur. Further, this lining up only happens within small domains of the metal having sizes in the range one tenth of a millimetre to a few millimetres. Within a domain the lining up is perfect but the different domains in a ferromagnetic material will generally have their directions of magnetization pointing in random directions as illustrated symbolically in figure 1.1(a). If they lined up
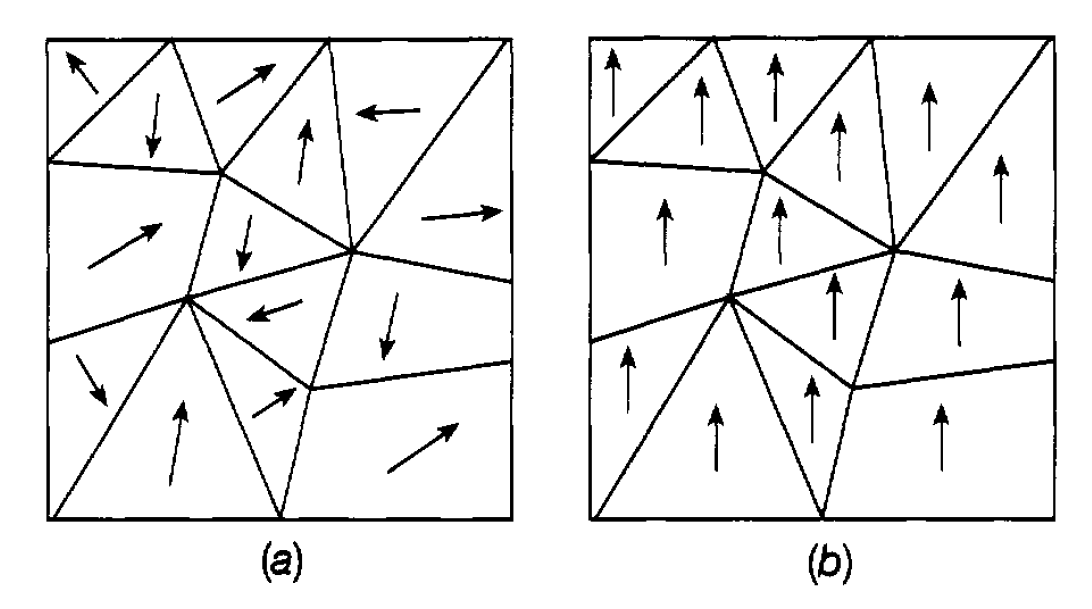
Figure 1.1: Domains in a ferromagnetic material: (a) unmagnetized, (b) magnetized.
throughout the material this would be a state of high energy because of the very large magnetic field caused by the domains all pointing in the same direction, and the system naturally tends to opt for lower-energy states. However, if the material is placed in an external magnetic field, then those domains pointing in the same direction as the field tend to grow and others rotate their direction of magnetization in the direction of the field (see figure 1.1(b)). The additional field due to the ferromagnet, which, of course, enhances the applied field, can be very strong indeed. If the applied field is then removed most of the domains will stay in position and we then have the well-known permanent magnet. This retained memory by the material of the direction of the original applied field is referred to as hysteresis. However this configuration is somewhat unstable and, for example, hitting a magnet with a hammer can reduce its magnetism the domains revert back to random orientations and lower energy.
Diamagnetism. Even if an atom does not behave as a small magnet, because all the electrons are in filled shells, there is still a small magnetic effect when a material formed from such atoms is placed in a magnetic field. In these atoms electrons in filled shells are moving although, as pointed out earlier in this section, their orbital rotations and the corresponding magnetic effects exactly cancel. However, because they are moving and carry an electric charge, when a magnetic field is applied they experience a force. This force modifies the motion of each electron with the consequence that the orbital rotations do not exactly cancel out. There is a net resultant angular momentum and this leads to a small magnetic effect. It turns out that the magnetic field due to this induced magnetism is always in the opposite direction to the inducing field and therefore reduces its effective size. Diamagnetism then acts in the opposite way to paramagnetism and ferromagnetism, which enhance the applied magnetic field. It will, of course, also arise in the filled shells of atoms with loose electrons but its effect is relatively very small compared with the magnetism due to the loose electrons and can generally be ignored. Unlike paramagnetism and ferromagnetism, it is also independent of temperature since thermal vibrations do not affect the motion of electrons within the atom, only the motion of the atoms as a whole.
 الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
الاكثر قراءة في مواضيع عامة في الفيزياء الصلبة
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















