Concentration/fractionation of proteins by salting out
Salting out using ammonium sulfate is one of the classical methods in protein biochemistry. Formerly it was widely used for the fractionation of proteins, but it is not a highly discriminating method and it is unusual to get a pure fraction, using this method. Today it is rather used as an inexpensive way of concentrating a protein extract, while leaving non-protein material in solution, and any purification with respect to protein is generally regarded as a bonus.
1. Why ammoniums sulfate?
Polyvalent anions are more effective at salting out than univalent anions, while polyvalent cations tend to negate the effect of polyvalent anions. The best combination is therefore a polyvalent anion with a univalent cation. Anions can be arranged in a so-called “Hofmeister series”, which describes their relative effectiveness in salting out at equivalent molar concentrations. In decreasing order of effectiveness, the series is: citrate > sulfate > phosphate > chloride > nitrate > thiocyanate. This series also describes a decreasing tendency for the anions to stabilize protein structure. Citrate and sulfate are thus “kosmotropes”, which tend to stabilize protein structure, while thiocyanate and nitrate are “chaotropes” which tend to destabilize protein structure. An ideal salt would, therefore, be citrate or sulfate combined with a univalent cation. Ammonium sulfate is most popular because it meets these criteria, is available in a pure form at low cost and is highly soluble, so that high solution concentrations can be attained. The sulfate ion has been viewed in a number of ways, regarding how it salts out proteins, including, ionic strength effects, kosmotropy, exclusion-crowding, dehydration, and binding to cationic sites, especially when the protein has a net positive charge (denoted ZH+). All of these may play a role, depending upon the salt concentration and the pH-dependent charge on the protein. Ionic strength effects. It will be noticed that the Hofmeister series goes from multivalent to univalent ions. This largely reflects the fact that the Hofmeister series is based on molarity, while ionic strength is a factor in salting out. The valency of the ion has an effect on ionic strength as can be illustrated by comparing NaCl with (NH4)2SO4.
Ionic strength is defined as:-

Where, ci = concentration of each type of ion (moles/liter)
Zi = charge of each type of ion.
Thus in the case of 1 M NaCl,
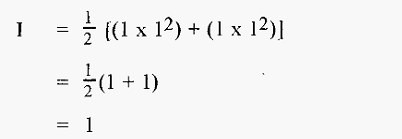
and for 1 M (NH4)2SO4,

Ionic strength effects come into play at low salt concentrations 0-0.2 M) and, as the name implies, they are not specific to ammonium sulfate. At low ionic strength, protein solubility is at its minimum at the proteins pI (Fig. 1). At this pH, intramolecular electrostatic forces between oppositely charged side chains are at a maximum, protein conformation is maximally tightened and protein hydration is least. On either side of the pI, titration of ionizable groups leads to a lessening of intramolecular ionic interactions. In consequence, protein structure becomes more relaxed and hydration and solubility are increased.
Addition of low concentrations of salt causes a similar weakening of intramolecular ionic bonds, with similar consequences of more relaxed protein structure and greater solubility. As shown in Fig. 1, addition of salt and altering of the pH, away from the pI, have similar, and additive, effects. The increase in solubility of protein upon addition of modest amounts of salt is known as “salting in”.

Figure 1. “Salting in” of proteins the interaction of pH and ionic strength (adapted from Dennison and Lovrien ).
Kosmotropy. At concentrations above 0.2 M the sulfate ion acts as a Hofmeister kosmotropes, i.e. it stabilizes protein structure, and concomitantly reduces its solubility. The effect of a kosmotropes, in stabilizing protein structure, can be described by the reaction:-
Relaxed, open protein structure ------ compact, tight structure (more soluble, less stable) (less soluble, more stable)
Kosmotropes may be described as “pushing” if they act on the left of this reaction and “pulling” if they act on the right, in either case driving the reaction to the right.
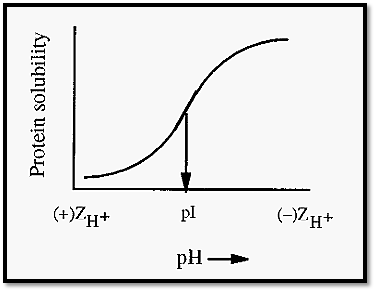
Figure 2. The effect of pH on the salting out of a protein by ammonium sulfate.
Sulfate can act as a pulling kosmotropes by virtue of its interaction with protein cationic sites. Consistent with this, the precipitation of proteins is usually promoted at pH values below the pI (Fig. 2), where the protein has a maximal number of cationic sites. Reinforcing its pulling effect is the fact that the sulfate ion is divalent, and so can bind to more than one cationic site at a time, and that it has a tetrahedral structure, with four oxygen atoms that can hydrogen bond to multiple sites on the protein.
Sulfate also acts as a pushing kosmotropes by virtue of its extraordinary hydration. By virtue of its hydration, the sulfate ion can act as a dehydrating agent and, in its hydrated form, as an exclusion-crowding agent. The sulfate anion has 13 or 14 water molecules in its first hydration layer and possibly more in a second layer. Consequently, in salting out at, say, 3 M ammonium sulfate, the sulfate anion will have accreted to itself 40 to 45 M out of the total of 55 M H2O in neat water. In salting out, therefore, a large proportion of the water will be involved in hydrating the sulfate ions and increasing their effective radius. The large, hydrated, [SO4.(H2O)n]2- ions crowd and exclude the proteins, pushing them into tighter, more ordered (less soluble) conformations, with lower entropy. The preferential accretion of the water molecules to the sulfate ions excludes the proteins from a proportion of the water (the proportion increasing with the salt concentration), ultimately bringing them to their solubility limit.
No other salt has the combination of properties which make ammonium sulfate so effective at salting out. Consequently, when the word salt is used in the context of salting out, it invariably means ammonium sulfate. Similarly, the term “ionic strength” is often used loosely, when what is really meant is the concentration of ammonium sulfate.
2 . Empirical observations on protein salting out.
Starting from zero, increases in salt concentration initially increase the solubility of the proteins, due to salting in. With further increases in ammonium sulfate concentration, the protein solubility passes through a maximum and then decreases (Fig. 3).
The salting out relationship is described by an empirical equation;-
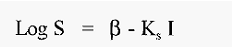
where,
S = protein solubility (g/l)
I = ionic strength
fl and KS are constants.
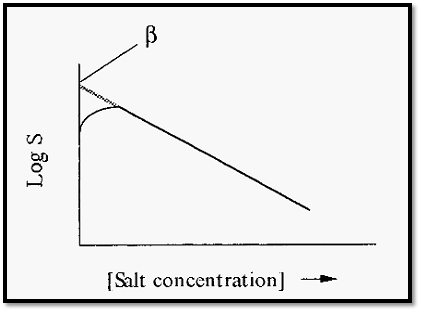
Figure 3. Solubility of a typical protein vs concentration of ammonium sulfate.
KS, the so-called “salting out constant” (the slope of the plot in Fig. 3), is essentially independent of temperature and pH but varies slightly with the nature of the protein. fl is markedly dependent upon the pH and temperature (Fig. 4) and also varies markedly with the nature of the protein.
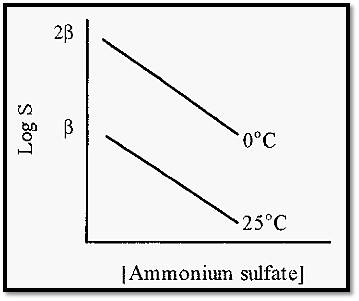
Figure 4. The effect of temperature on the salting out of carboxy haemoglobin.
Note that a rise in temperature causes a decrease in fl. Note also that, since fl is in units of log S, a unit change in fl represents a ten-fold change in solubility. Therefore, a protein will be markedly less soluble at higher temperatures and in practice it is better to conduct salting out at, say, 25C rather than at 4C. The sulfate ion is kosmotropic, so proteins are stabilized by the presence of (NH4)2SO4 and a high salt concentration also inhibits microbial growth. For these reasons, also, it is less necessary than usual to work at a low temperature.
The initial concentration of a protein in solution has a major influence on the amount of (NH4)2SO4 required to precipitate it. Proteins appear to fall into two categories, denoted type I and type II, depending upon how their concentration affects their salting out behaviour. For type I proteins, each protein has a characteristic precipitation curve (e.g. Fig. 5).
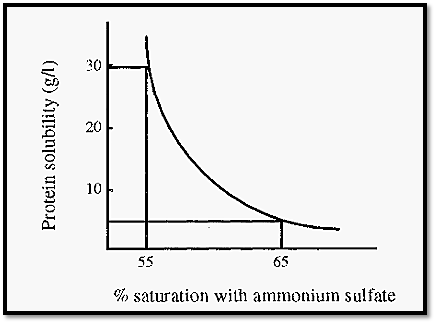
Figure 5. The salting out curve of carboxymyoglobin .
The lower the concentration of protein in solution, the more salt is required to precipitate it. In the example given in Fig. 5, if carboxymyoglobin is present at an initial concentration of 30 g/l, it will begin to precipitate at about 55% saturation with (NH4)2SO4, whereas at an initial concentration of 4 g/l, 65% saturation is required to begin precipitation.
Not all proteins behave in this simple way. Type II proteins2, such as BSA and α -chymotrypsin, precipitate to an extent dependent upon their initial concentration, i.e. such proteins manifest a family of precipitation curves, each curve arising from a particular initial protein concentration (Fig. 6).
Type I proteins have a single precipitation curve, regardless of the initial protein concentration. Type II proteins precipitate in a manner dependent upon their initial concentration.

Figure 6. Differential salting out behaviour of type I and type II proteins.
Clearly, therefore, proteins do not precipitate between fixed and characteristic limits of ammonium sulfate concentration, as is implied in much of the older literature. Also, there is little point in repeating a precipitation, from the same volume and at the same ammonium sulfate saturation. Since the first precipitation will not have been quantitative, the concentration will be less if the protein is reconstituted in the same volume. To repeat the precipitation, the protein concentration should be readjusted to the same value as previously, which is not always practicable. In general, it is not worth repeating the precipitation as the cost, in terms of protein lost, is not justified by the increase in protein purity obtained.
Proteins may be purified from a mixture by differential precipitation at different saturations of ammonium sulfate (e.g. Fig. 7). The protein solubility curve (Fig. 7) has two steps, due to the precipitation of the serum albumin, followed by the carboxymyoglobin. Such perfect separation is rare, however, and it is more usual to obtain mixed fractions with, possibly, only slight enrichment of a desired protein. By altering the protein concentration it is sometimes possible to improve the separation, e.g. by diluting the solution, the points of precipitation (the peaks in the first derivative curve) will be moved to the right, to higher saturation levels. Due to differences in KS, however, the peaks due to different proteins might move to different extents and the separation will thus be improved.
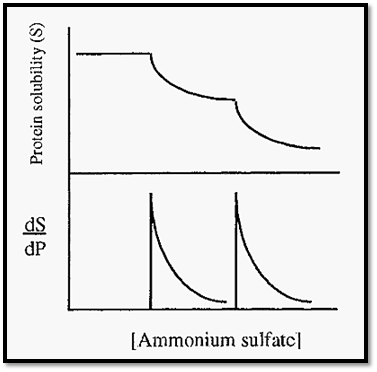
Figure 7. Separation of human serum albumin and carboxymyoglobin by salting out .
To summarize, in salting out the following can be manipulated;-
• pH. It is best to use a pH below the pI of the desired protein where
precipitation is maximal.
• Temperature. Theoretically, the best temperature is the highest temperature at which the protein is stable. fl decreases as the temperature is increased and there is therefore greater precipitation at higher temperatures. For practical purposes, room temperature (25C) is adequate.
• Protein concentration. The difficulty here is that the direction of any effects cannot be predicted in advance.
The resolving power of salting out is not high and so it is now commonly used mainly as a means of concentrating proteins from dilute extracts, while leaving non-protein molecules in solution. It is also generally used early in an isolation, immediately after preparation of the extract.
3. Three-phase partitioning (TPP)
Three-phase partitioning (TPP) is a method in which proteins arc salted out from a solution containing a mixture of water and t-butano. t-Butanol is infinitely miscible with water but upon addition of sufficient ammonium sulfate the solution splits into two phases, an underlying aqueous phase and an overlying t-butanol phase. If protein was present in the initial solution, three phases would be formed, protein being precipitated in a third phase between the aqueous and t-butanol phases (Fig. 8). The amount and type of protein precipitated is dependent upon the ammonium sulfate concentration, as in conventional salting out. Unlike in conventional salting out, however, the protein precipitate is largely dehydrated and has a low salt content. Desalting before a subsequent ion-exchange step, which is a time-consuming necessity after conventional salting out, is therefore generally not necessary with TPP.
Conventional salting out is effected by adding (NH4)2SO4 to a purely aqueous solution of protein. In this case the protein is initially hydrated and is thus soluble, and the addition of the salt, serves to dehydrate the protein and eventually brings it to its solubility limit.

Figure 8. Three-phase partitioning.
In TPP, t-butanol may be first added to the aqueous solution of protein to about 20%. It is believed that this results in the protein equilibrating with the solvent (water) and the co-solvent (t-butanol). The protein thus becomes partially hydrated and partially t-butanolated, in proportion to the relative abundance of the solvents in the mixture.
Upon addition of (NH4)2SO4, water is abstracted by the salt ions as these become hydrated. The salt apparently has a higher affinity for water than for t-butanol, and thus preferentially sequesters the water. In the absence of protein, this results in the solution dividing into two phases, as some of the water is made “unavailable” to the t-butanol. If protein is present, the protein equilibrates with the new proportions of solvent and co-solvent available to it. Upon addition of further (NH4)2S04, eventually the amount of water available to the protein becomes insufficient to keep the protein in solution, and it precipitates.
At this point, however, the protein will be largely t-butanolated. This results in the protein having a reduced density and so, when it precipitates with the situation in conventional salting out where the dehydrated protein normally sinks, indicating that it is more dense than the solution.
In conventional salting out, as the concentration of (NH4)2SO4 increases, the solution density increases and the difference in density between the solution and the precipitate decreases, until the point is reached where it is no longer possible to sediment the precipitate. In the case of TPP, however, the precipitate is less dense than the solution and so, with increasing (NH4)2SO4 concentration, the precipitate floats more and more easily.
In non-aqueous environments, a-helices are favoured and so, during TPP, as the proportion of t-butanol increases, the protein conformation may become distorted as it acquires a greater proportion of a-helices. This distortion leads to the denaturation of many proteins, which may be a disadvantage. On the other hand, if the protein of interest is able to survive TPP, then it is likely that TPP will effect a purification by the denaturation of impurities, as well as by its fractionating ability.
Selective denaturation of contaminating protein has been put to good effect in the isolation of cathepsin D10 and a number of erythrocyte proteins", where the major problem was the presence of a large excess of haemoglobin.
To effect a fractionation of a protein mixture by TPP, about 20% t
butanol can be added to the protein mixture in aqueous solution and increments of (NH4)2SO4 are added, the interfacial precipitate being concentrated by centrifugation and removed for analysis after each increment of (NH4)2SO4.
Empirically, it has been found that, in TPP;-
• Proteins precipitate in order of their molecular weight, i.e. larger proteins precipitate before smaller proteins, present at the same concentration.
• Proteins are most readily precipitated into the third phase when they
have a positive charge,
• Proteins are most soluble, after TPP (i.e. denaturation is minimal), when TPP is done at the pI of the protein.
• The protein concentration has a marked influence: generally the greater the concentration, the more easily the protein will precipitate.
• Temperature has little effect, in the range 0C to 25C.
It should be noted that t-butanol is unusual in that it is an organic solvent which tends not to denature proteins. TPP can therefore be done at room temperature, which is fortunate because t-butanol solidifies at about 25C, and is most conveniently used above this temperature.
References
Dennison, C. (2002). A guide to protein isolation . School of Molecular mid Cellular Biosciences, University of Natal . Kluwer Academic Publishers new york, Boston, Dordrecht, London, Moscow .
 الاكثر قراءة في عزل البروتين
الاكثر قراءة في عزل البروتين
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


