


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-7-2016
Date: 8-1-2020
Date: 8-7-2018
|
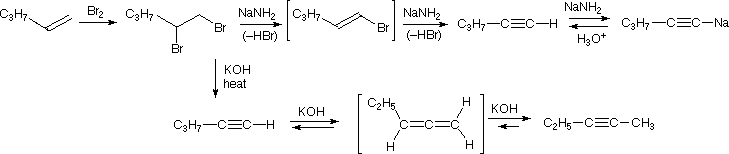
The acidity of terminal alkynes also plays a role in product determination when vicinal (or geminal) dihalides undergo base induced bis-elimination reactions. The following example illustrates eliminations of this kind starting from 1,2-dibromopentane, prepared from 1-pentene by addition of bromine. The initial elimination presumably forms 1-bromo-1-pentene, since base attack at the more acidic and less hindered 1 º-carbon should be favored. The second elimination then produces 1-pentyne. If the very strong base sodium amide is used, the terminal alkyne is trapped as its sodium salt, from which it may be released by mild acid treatment. (NB One cannot stop the reaction at the terminal alkyne with 2 equivalents of strong base.) However, if the weaker base KOH with heat is used for the elimination, the terminal alkyne salt is not formed, or is formed reversibly, and the initially generated 1-pentyne rearranges to the more stable 2-pentyne via an allene intermediate.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|