
Translational Kinetic Energy
 المؤلف:
Professor John W. Norbury
المؤلف:
Professor John W. Norbury
 المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
المصدر:
ELEMENTARY MECHANICS & THERMODYNAMICS
 الجزء والصفحة:
p 231
الجزء والصفحة:
p 231
 1-1-2017
1-1-2017
 2731
2731
Translational Kinetic Energy
For a single molecule its average kinetic energy is
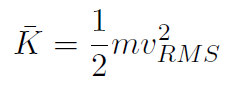
and using 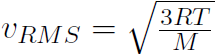 gives
gives 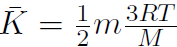 .
.
Remember that M is the molar mass, which is the mass of 1 mole of gas and m is the mass of the molecule. Thus 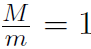 mole = 6.02 ×1023 = NA, Avagadro's number. Thus
mole = 6.02 ×1023 = NA, Avagadro's number. Thus 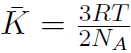 or
or
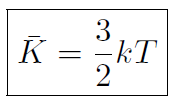
This is a very interesting result. For a given temperature T, all gas molecules, no matter what their mass, have the same average translational kinetic energy.
Example In the center of the Sun the particles are bare hydrogen nuclei (protons). Calculate their average kinetic energy.
Solution The center of the Sun is at a temperature of about 20,000,000oK. Thus
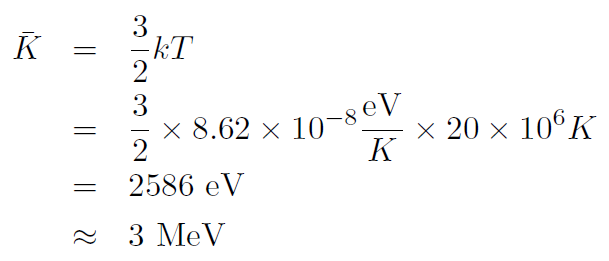
 الاكثر قراءة في الميكانيك
الاكثر قراءة في الميكانيك
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


