

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The reaction Gibbs energy
المؤلف:
Peter Atkins، Julio de Paula
المصدر:
ATKINS PHYSICAL CHEMISTRY
الجزء والصفحة:
201
2025-11-17
244
The reaction Gibbs energy
Consider the equilibrium A 5 B. Even though this reaction looks trivial, there are many examples of it, such as the isomerization of pentane to 2-methylbutane and the conversion of l-alanine to d-alanine. Suppose an infinitesimal amount dξ of A turns into B, then the change in the amount of A present is dnA =−dξ and the change in the amount of B present is dnB =+dξ. The quantity ξ (xi) is called the extent of reaction; it has the dimensions of amount of substance and is reported in moles. When the extent of reaction changes by a finite amount ∆ξ, the amount of A present changes from nA,0 to nA,0 −∆ξ and the amount of B changes from nB,0 to nB,0 +∆ξ. So, if initially 2.0 mol A is present and we wait until ∆ξ =+1.5 mol, then the amount of A remaining will be 0.5 mol. The reaction Gibbs energy, ∆rG, is defined as the slope of the graph of the Gibbs energy plotted against the extent of reaction:
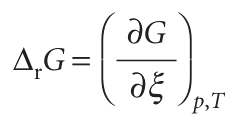
slope of G with respect to ξ. However, to see that there is a close relationship with the normal usage, suppose the reaction advances by dξ. The corresponding change in Gibbs energy is
dG=µAdnA+µBdnB=−µ dξ+µBdξ=(µB−µA) dξ
This equation can be reorganized into
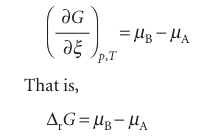
We see that ∆rG can also be interpreted as the difference between the chemical potentials (the partial molar Gibbs energies) of the reactants and products at the com position of the reaction mixture. Because chemical potential varies with composition, the slope of the plot of Gibbs energy against extent of reaction changes as the reaction proceeds. Moreover, because the reaction runs in the direction of decreasing G (that is, down the slope of G plotted against ξ), we see from eqn 7.2 that the reaction A → B is spontaneous when µA > µB, whereas the reverse reaction is spontaneous when µB > µA. The slope is zero, and the reaction is spontaneous in neither direction, when , ∆rG =0 , This condition occurs when µB = µA (Fig. 7.1). It follows that, if we can find the composition of the reaction mixture that ensures µB = µA, then we can identify the composition of the reaction mixture at equilibrium.
 الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
الاكثر قراءة في مواضيع عامة في الكيمياء الفيزيائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















